CAR-T (Chimeric Antigen Receptor T-Cell), as an immunocytotherapy regimen, has attracted a lot of attention from academics, doctors, patients, and investors worldwide. What does make it so attractive and popular?
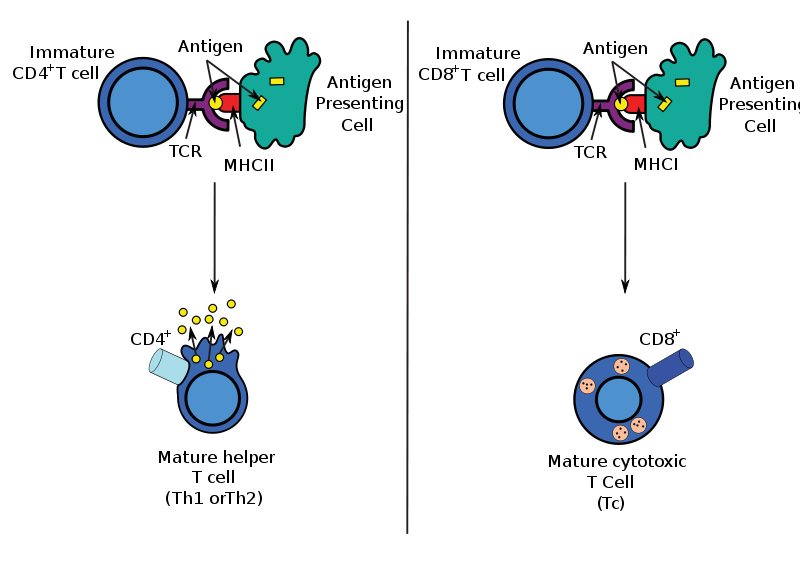
CAR-T modifies human T cells by genetic engineering to express chimeric antigen receptor (CAR), linking antibodies that recognize a tumor cell surface antigen and signal molecules that activate T cells, to enhance specific killing effects of T cells on tumors.
The chimeric antigen receptor (CAR) is introduced into T cells to produce tumor-specific T cells. Once T cells express this receptor, a single fusion molecule can specifically bind to the antigen and activate T cells. Most of the CARs consist of an antigen-binding region, an extracellular region, a transmembrane region and an intracellular signal region capable of activating T cells after binding to the antigen. Previous studies showed that the single chain antibody fragment (scFv) expressed by T cells can regulate the function and specificity of T cells when combining with the T cell signaling region. Advances in transformation and amplification technology of tumor-specific human T cells make this technique a potential therapeutic strategy.
CAR recognizes tumor antigens in an HLA-independent manner, allowing cells that have been modified by CAR to down-regulate this tumor cell immune escape mechanism across HLA. And due to the non-HLA-dependence of tumor recognition, CAR application can break through the limitations of patients with HLA typing.CAR-modified T cells can target polyclonal antibodies against any cell surface antigen, including protein, sugar, and glycolipids, which allows CAR-modified T cells to recognize a wider range of targets than native T cell surface receptor (TCRs). CAR transformation can regulate most T cell subsets, including CD4, CD8, memory T cells or effector T cells. The transformation of CAR can carry one or more T cell activation signals to improve the ability of T cells to proliferate, survive and cleave the target cells. Moreover, T cell genetics transformation not only helps activate signals but also to design T cells carrying potential anti-cancer immune regulatory factors (such as cytokines) to enhance the anti-tumor effect in the tumor microenvironment. The last and most important thing is that this technique can produce a large number of T cells with tumor killability in a relatively short period of time, making this technique clinically possible.
CAR’s underlying design includes a tumor-associated antigen (TAA) binding region, an extracellular hinge region, a transmembrane region, and an intracellular signal Area. The choice of target antigen is a key determinant of CAR specificity, efficacy, and the safety of genetically engineered T cells. The ideal target antigen is only expressed in tumor cells and is important for the survival of tumor cells. Unfortunately, most of the expressed TAAs do not have tumor cell restriction, leading to “production of targeted / non-tumor” toxicity. Historically, CAR-modified T cells only target the cell surface antigen.
Currently, T cells are widely used to express the CD19 antibody, which is expressed not only on all normal B cell surfaces but also on the surface of most malignant proliferative B cells. Since CD19 is not expressed in hematopoietic stem cells, its toxicity is also limited to B-cell aplastic anemia when treated with anti-CD19 CAR-T cells. There are other CAR-T targeting molecules targeting ERRB2 (HER-2 / neu) for the treatment of lung and prostate cancer, prostate-specific antigens for prostate cancer, CAIX for renal cell carcinoma, Lewis Y for the treatment of lung cancer and ovarian cancer and so on, proving that CAR-T cells are widely used in anti-tumor applications.
Although most of the CAR T scFV are used to target tumors, a part of CARs take advantages of receptor ligands fusing the transmembrane signal region, including anti-VEGFR2,anti-avb6,anti-Her3/4 receptor etc. The improvement in CAR design and the activation of T cells are all involved in the enhancement of the affinity of the antibody recognition region. The improvement of CAR antigen affinity also enhances the ability of tumor targeting with the downregulation of antigen expression and the increase of T cell activation.