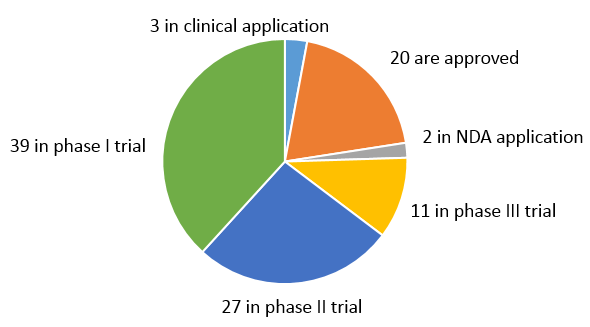
1. There are 102 EGFR-targeted drugs that are approved globally and in clinical research in total.
Up to now, there are 20 EGFR-targeted drugs approved by the global market (Europe, USA, Japan, South Korea, etc.), 80 in clinical application and research stage, and 2 in NDA application.
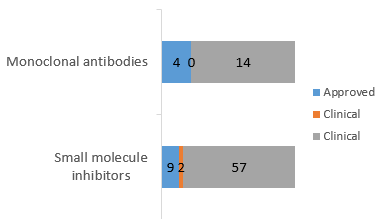
2. EGFR-targeted drugs are mainly small molecule inhibitors.
Molecular targeting drugs developed for EGFR are divided into two major categories:
1) There are 18 monoclonal antibodies, such as cetuximab and panitumumab. They can bind to EGFR extracellular region and block ligand-dependent EGFR activation.
2) There are 68 small molecule inhibitors, including small molecule multi-target receptor tyrosine kinase (RTKs) Inhibitors and small molecule tyrosine kinase inhibitors (EGFR-TKls), which can inhibit tyrosine kinase’s activity in EGFR intracellular region.
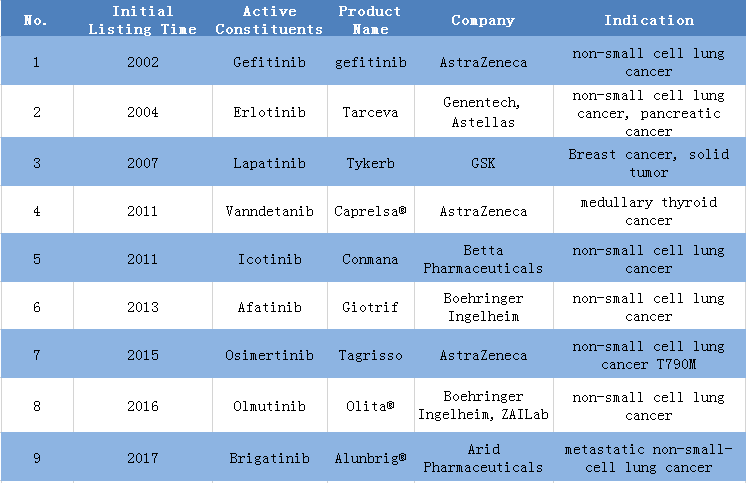
3. 9 EGFR small molecule inhibitor drugs are approved.
1) AstraZeneca has 3 approved drugs, occupying one third of the global market.
2) The approved EGFR small molecule inhibitors are mainly sued for the treatment of non-small cell lung cancer.
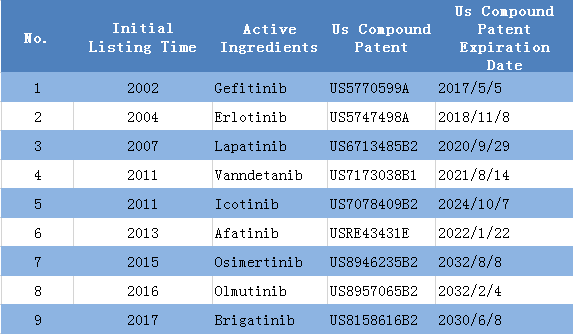
3) In the United States, there are 8 approved EGFR small molecule inhibitor drugs which have patent protection. Gefitinib is confronting with patent cliff.
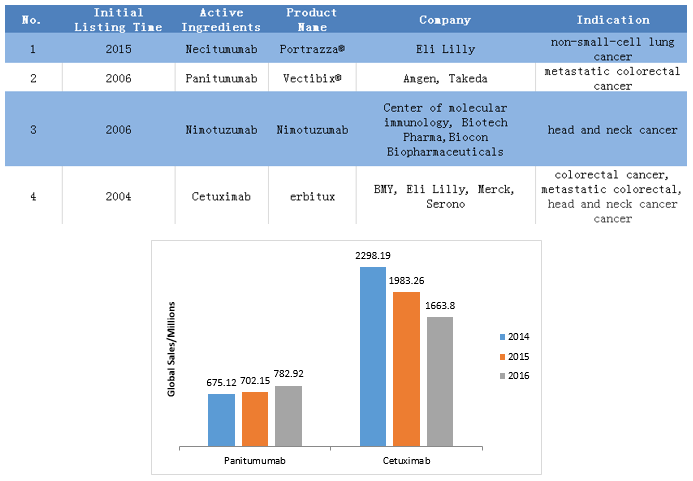
4. 4 EGFR-targeted monoclonal antibodies are approved.
1) Antibody class EGFR-targeted drugs are mainly used for the treatment of rectal cancer, head and neck cancer.
2) Eli Lilly has 2 approved drugs, dominating the EGFR monoclonal antibody market.
3) Cetuximab is the champion of the year, with sales twice as much as panitumumab of the same indication.
Notes: The global sales showed in the histogram are the original sales of the relevant company. The above data was retrieved on July 12, 2017.