October 03, 2018 – Seattle Genetics and Takeda recently announced the evaluation of antibody conjugates Adcetris (brentuximab vedotin) for the treatment of peripheral T-cell lymphoma (PTCL, also known as mature Phase III clinical study of T-cell lymphoma, MTCL) reached the primary endpoint of ECHELON-2. Based on data of the research, Seattle Genetics has planned to submit Adcetris’ Supplementary Biological License (sBLA) to the US FDA in the near future.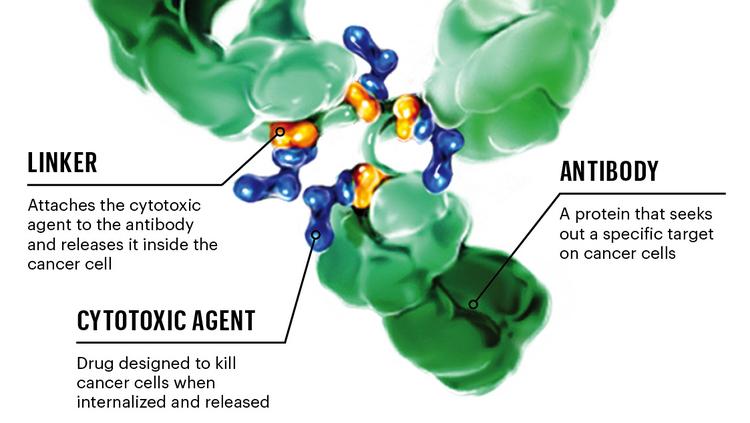
PTCL Clinal Program Introduction
ELON-2 is the largest randomized, double-blind, phase III study to date in PTCL patients enrolled in previously untreated CD30-positive PTCL patients, which evaluated for Adcetris in combination with chemotherapy regimen CHP (cyclophosphamide + A) and the efficacy and safety of CHOP (cyclophosphamide + doxorubicin + vincristine + prednisone) in first-line treatment compared to the currently accepted first-line standard care regimen for PTCL. The primary endpoint of the study was progression-free survival (PFS) assessed by the Independent Review Body (IRF).
The results of the study showed that the Adcetris+CHP regimen achieved a statistically significant improvement in PFS (HR=0.71, p=0.0110) compared to the CHOP regimen, reaching the primary endpoint of the study. In addition, the Adcetris+CHP regimen also demonstrated superiority in key secondary endpoint overall survival (OS) compared to the CHOP regimen (HR=0.66, p=0.0244). In other key secondary endpoints, the Adcetris+CHP regimen also showed statistically significant advantages, including: PFS, complete response rate (CRR), and objective response rate (ORR) in patients with intersystem degenerative large cell lymphoma (sALCL). In this research, the Adcetris+CHP scheme has comparable security to the CHOP scheme. Detailed data will be announced at the annual meeting of the American Society of Hematology (ASH) in early December.
Antibody-drug Conjugate (Adcetris)
Adcetris is an antibody-conjugated drug (ADC) that is coupled by a monoclonal antibody that targets the CD30 protein and a microtubule disrupter (monomethyl auristatin E, MMAE) via a protease-sensitive crosslinker. The coupling technology is the proprietary technology of Seattle Genetics. CD30 protein is a clear marker of classical Hodgkin’s lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL), while Auristatin E blocks cell division by inhibiting the polymerization of tubulin. Adcetris is stable in the blood and releases MMAE after internalization by CD30-positive tumor cells.
Adcetris was developed by Seattle Genetics, and in 2009, Takeda reached a licensing agreement and obtained commercial rights to the drug in other countries except the US and Canada. By far, Adcetris has been approved by more than 70 countries around the world.
Currently, Takeda and Seattle Genetics are actively promoting a large phase III clinical development project to make Adcetris as a basic care drug for the treatment of CD30-positive lymphoma and to redefine the clinical first-line treatment of lymphoma.
