The field of antibody-drug conjugates (ADCs) is experiencing a major transformation due to the development of bispecific ADCs (BsADCs) that merge the dual-targeting functionality of bispecific antibodies (BsAbs) with the cell-killing power of standard ADCs. This approach overcomes key problems in traditional ADCs including inadequate internalization and off-target effects along with drug resistance while enabling new treatment options. This section investigates the scientific foundation and examines both current progress and future opportunities for BsADCs in cancer therapy.
1. The Rationale Behind Bispecific ADCs
1.1 Dual-Targeting Mechanisms
BsADCs are engineered to bind two distinct antigens or epitopes on a single tumor cell. This dual specificity enhances tumor selectivity by requiring simultaneous engagement of both targets for internalization, thereby reducing off-target effects in healthy tissues. For example, a BsADC targeting HER2 and LAMP3 promotes receptor clustering, leading to rapid lysosomal trafficking and payload release, a mechanism shown to outperform monospecific ADCs in preclinical models.
1.2 Enhanced Internalization and Payload Delivery
A key challenge with traditional ADCs is inefficient internalization, particularly for antigens with low expression or slow recycling rates. BsADCs overcome this by leveraging dual-target engagement to trigger robust receptor internalization. Studies demonstrate that BsADCs targeting PRLR and HER2 achieve up to 100-fold improvements in internalization efficiency compared to monospecific counterparts, significantly enhancing cytotoxic payload delivery.
1.3 Overcoming Resistance
Tumor heterogeneity and antigen downregulation often lead to ADC resistance. BsADCs mitigate this by targeting two antigens simultaneously, reducing the likelihood of escape mutations. For instance, a BsADC targeting MET via two distinct epitopes induces 2:2 antigen-antibody complexes, promoting sustained internalization even in MET-low tumors.
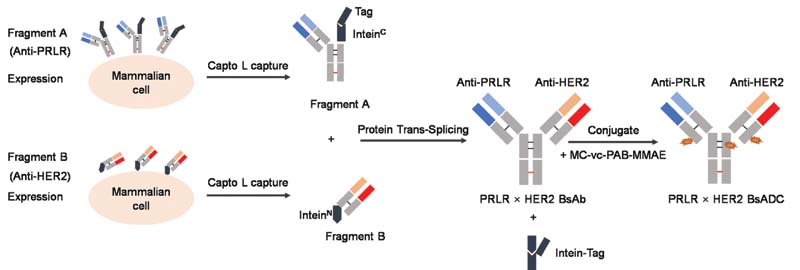
Figure 1. Schematic representation of fragment expression, the split intein trans-splicing, and drug conjugation process used to generate reactivated BsADC. 1
2. Recent Scientific Breakthroughs (2023–2024)
2.1 Novel Structural Designs
Recent innovations focus on optimizing BsAb formats for ADC conjugation. IgG-like BsAbs with asymmetric Fc regions are gaining traction due to their extended half-life and improved manufacturability. For example, a tetravalent HER2-targeting BsADC (binding two HER2 epitopes) demonstrated potent activity in esophageal and lung cancer models, though clinical translation requires balancing efficacy and toxicity.
2.2 Payload and Linker Innovations
Next-generation BsADCs incorporate site-specific conjugation technologies to ensure homogeneous drug-to-antibody ratios (DARs). A novel approach using non-natural amino acids enables precise coupling of microtubule inhibitors (e.g., hemiasterlin derivatives) or DNA-damaging agents, achieving DARs of 4–8 without compromising stability. Cleavable linkers responsive to tumor-specific proteases further enhance payload release specificity.
2.3 Preclinical and Clinical Highlights
- Dual-Targeting HER2: A BsADC targeting two HER2 epitopes (ZW49) showed synergistic effects in gastric and breast cancer models by inducing unique HER2 clustering and complement-dependent cytotoxicity.
- EGFR/c-MET BsADC: This candidate, currently in Phase I trials, addresses resistance to EGFR inhibitors by co-targeting c-MET, a bypass signaling pathway in non-small cell lung cancer (NSCLC).
- FRα/TROP2 BsADC: Preclinical data revealed potent activity in ovarian and triple-negative breast cancers, with a 33% response rate in FRα-high tumors.
3. Technical Challenges and Solutions
3.1 Structural Complexity
BsADCs face hurdles in antibody assembly, particularly chain mispairing in IgG-like formats. Solutions include knob-into-hole engineering and scFv-Fc fusions to ensure correct heavy/light chain pairing. For example, GenScript’s platform uses recombinant DNA technology to co-express two distinct heavy and light chains in a single cell line, minimizing non-functional variants.
3.2 Pharmacokinetic Optimization
Smaller BsAb fragments (e.g., BiTE-based BsADCs) exhibit rapid clearance, limiting their utility. Strategies like PEGylation or Fc fusion extend half-life while retaining tumor penetration.
3.3 Toxicity Management
Dual targeting may increase on-target toxicity if antigens are expressed in healthy tissues. Affinity tuning—reducing binding strength for the less critical target—has proven effective. For instance, a BsADC targeting EGFR (low affinity) and c-MET (high affinity) minimized EGFR-driven toxicity while maintaining antitumor activity.
4. Clinical Pipeline and Future Directions
4.1 Promising Candidates in Trials
Over 15 BsADCs are in clinical development globally, targeting cancers such as NSCLC, ovarian, and head/neck squamous cell carcinoma (HNSCC). Notable examples include:
- EGFR/HER3 BsADC: In Phase III for NSCLC, this candidate demonstrated a 34% objective response rate in EGFR TKI-resistant patients.
- MET×MET BsADC: A Phase I/II trial (NCT04982224) is evaluating its safety in MET-overexpressing tumors, with preclinical data showing complete tumor regression in xenograft models.
4.2 Synergy with Immunotherapy
Emerging combinations with PD-1/PD-L1 inhibitors aim to enhance immune activation. For example, an anti-HER2/TLR8 BsADC (SBT6050) combines cytotoxic payloads with TLR8 agonists to stimulate dendritic cells, creating an “immunogenic cell death” effect.
4.3 Market and Research Outlook
The global BsADC market is projected to reach $70 billion by 2033, driven by advancements in targeting and payload technologies. Future research will focus ontumor-agnostic BsADCs (targeting pan-cancer antigens like CLDN6) and tri-specific ADCs incorporating immune-modulating domains.
5. Conclusion
BsADCs create a transformative approach to ADC therapeutics which solves persistent precision oncology problems. These molecules demonstrate potential to treat challenging cancers because they merge dual-targeting precision with improved delivery of therapeutic payloads. Following the progression of clinical trials BsADCs will establish themselves as foundational treatments in future cancer therapy.
Accelerate Your ADC Development with Creative Biolabs
Creative Biolabs develops advanced bispecific antibody-drug conjugates (ADCs) which enhance both therapeutic accuracy and effectiveness. Explore our specialized services:
Bispecific ADCs Development: Our team develops tailored bispecific ADC designs by combining sophisticated engineering solutions with therapeutic knowledge. Learn more about our bispecific ADCs development.
Fast-Internalizing Receptor-Based ADCs: The focus of our platform is on receptor-based bispecific ADCs that internalize quickly to facilitate rapid cellular uptake and improved drug delivery performance. Discover our approach to fast-internalizing receptor strategies.
c-MET-Targeted Bispecific ADCs: Precision-engineered bispecific ADCs for cancer treatment primarily aim to target the c-MET receptor. Explore our c-MET-based bispecific ADCs.
Biparatopic HER2-Targeted ADCs: Our implementation of biparatopic targeting enhances binding strength and therapeutic performance of ADCs that focus on HER2. Explore our bispecific ADCs which utilize biparatopic methods to target HER2.
CTLA-4-Based Bispecific ADCs: Our CTLA-4-directed bispecific ADCs regulate immune responses while providing immunotherapy treatment alternatives. Learn about our CTLA-4-targeted innovations.
BCMA-Targeted Bispecific ADCs: Our emphasis on BCMA enables us to develop bispecific ADCs which deliver precise treatment alongside improved therapeutic outcomes for patients with multiple myeloma. Read more about our BCMA-based bispecific ADCs.
Creative Biolabs drives ADC development by forming strategic partnerships which utilize innovative methods and precision engineering. You can count on us to turn your therapeutic vision into reality.
Reference
- Hui-Fang Zong, Bao-Hong Zhang. et a Generating a Bispecific Antibody Drug Conjugate Targeting PRLR and HER2 with Improving the Internalization. Pharmaceutical Fronts 2022; 04(02): e113-e120. https:doi.org/10.1055/s-0042-1749334. Distributed under Open Access license CC BY 4.0, without modification.