In a new study, researchers from Yale School of Medicine hide tumor-fighting antibodies from cancer by disguising them in molecules that cancers use to nourish tumor growth, based on a new therapy from the Yale Cancer Center. defense system. This Trojan horse therapy has been shown to be effective in the laboratory against several cancer tumor types, including brain tumors that are difficult for drugs to reach because of the protection of the blood-brain barrier. Relevant research results were published online in the journal ACS Central Science on July 15, 2024, with the title “Cathepsin B Nuclear Flux in a DNA-Guided ‘Antinuclear Missile’ Cancer Therapy”.
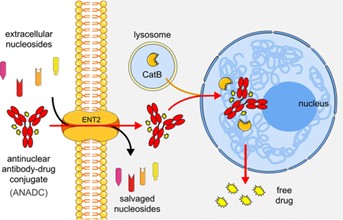
Fig. 1 The mechanism diagram of DNA-guided “antinuclear missile” cancer therapy.1
These successes are attributed to drug-carrying antibodies strategically redesigned from lupus to exploit their ability to target tumors while eliminating their effects on lupus. Scientists call such antibodies “antinuclear antibodies”, and they secretly hitch a ride on nucleic acid molecules that cancers pick up from the environment and use to synthesize new DNA to help tumors grow. Once inside the tumor, they effectively drop their camouflage and fire their anti-nuclear missile cargo to kill the cancer cells.
Unlike other treatments that pair traditional antibodies with chemotherapy, the antibodies in this therapy don’t target cells by circulating through the bloodstream and looking for tumor cell surface markers, such as HER2 or PD-L1, but instead unknowingly target them. into the tumor environment. One promising effect that may arise from this very targeted therapy is that patients experience fewer toxic side effects.
“Instead, the antinuclear antibody-drug conjugate (ANADC) looks for peptides floating around the tumor,” said James Hansen, corresponding author of the paper, a member of the Yale Cancer Center and director of radiation oncology at the Yale Gamma Knife Project. DNA fragments, meaning ANADC can track them even if tumors lack specific surface receptors that would prevent other, more traditional antibodies from finding them.”
Hansen said ANADC was effective in mouse models of breast and colon cancer and even improved survival in mouse models of glioma. The authors are currently working to advance this therapy into clinical trials.
“By targeting extracellular nucleic acids rather than surface receptors, ANADC can target essentially any type of necrotizing tumor, making it a tumor diagnostic therapy” and therefore has potential for use in other diseases, Hansen said.
“This technology gives us the opportunity to use antinuclear antibodies to deliver drugs, proteins, or gene therapies to tumors or other sites of damage associated with increased DNA release, such as heart disease, stroke, or trauma,” Hansen said.
View our comprehensive ADC services:
- ADC Antibody Screening
- DrugLnk™ Custom Synthesis
- Antibody Design and Conjugation
- ADC In Vitro Analysis
- ADC In Vivo Analysis
- ADC Manufacturing
Reference
- Cao, Fei, et al. “Cathepsin B Nuclear Flux in a DNA-Guided “Antinuclear Missile” Cancer Therapy.” ACS Central Science(2024).