Bacterial infections are one of the most serious threats to human health, causing millions of deaths worldwide every year. However, due to the emergence of bacterial resistance, the antibacterial activity of many traditional antibiotics against many diseases caused by bacteria is weakened or even almost lost. In addition, the formation of bacterial biofilms, a group of microorganisms enclosed in an extracellular matrix, the main components of which are proteins and polysaccharides, undoubtedly exacerbates the current unoptimistic treatment situation. Therefore, research and development of new highly effective antibacterial drugs and new antibacterial strategies are of great significance for humans to get rid of this dilemma.
Fortunately, there are many potential antibacterial agents under research, such as cationic photosensitizers, metabolic probes, metal nanomaterials, antimicrobial peptides (AMPs) and cationic polymers. As a new type of antibacterial material, amp has many advantages. Antimicrobial peptides, as an important component of the innate immune system of many animals and plants in the ecological environment, exhibit excellent antibacterial activity against both Gram-negative bacteria (Ge) and Gram-positive bacteria (Gþ). Its most widespread mode of action is to increase permeability or disrupt bacterial membranes, leading to extravasation of bacterial contents and bacterial death. Because of this, and because AMPs work differently than traditional antibiotics, it’s nearly impossible for bacteria to become resistant to them.
HHC10 (KRWWKWIRW) is a peptide screened by an artificial neural network. It has certain antibacterial activity against Ge and G+ bacteria. Its antibacterial activity mainly comes from the destruction of bacterial membranes. Antimicrobial peptides have broad-spectrum antibacterial activity and are not prone to bacterial resistance, making them one of the promising antibacterial drugs that have been widely explored in recent years.
Recently, researchers from Xiangya School of Pharmacy, Central South University, published an article titled “Harnessing antimicrobial peptide-coupled photosensitizer to combat drug-resistant biofilm infections through enhanced photodynamic therapy” in the journal Acta Pharm Sin B. This study reveals that it has conjugation of antimicrobial peptides with membrane-disrupting properties, and photosensitizers is a novel and effective strategy for treating bacterial infections.
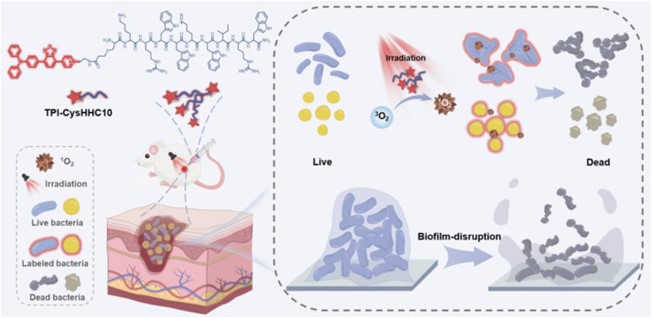
Bacterial biofilm-related infections are one of the most serious threats to human health. However, effective drugs against drug-resistant bacteria or biofilms are rarely reported. Here, we propose an innovative strategy to develop multifunctional antibacterial agents with broad-spectrum antibacterial activity by conjugating photosensitizers (ps) with antimicrobial peptides (amps).
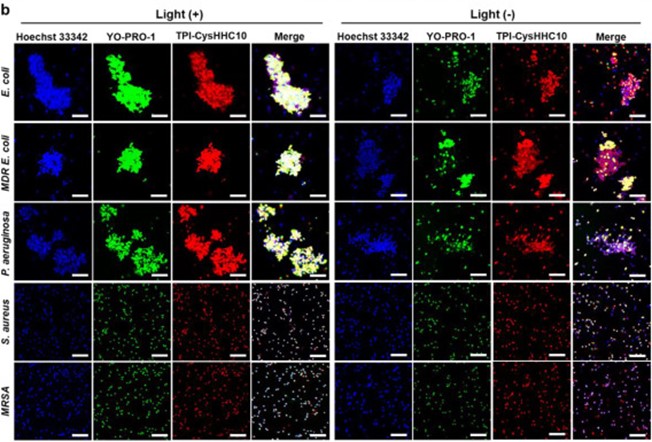
Fig. 1 CLSM images of E. coli, MDR E. coli, P. aeruginosa, and MRSA after incubation with TPI-CysHHC10.1
This strategy utilizes the ability of photosensitizers to generate reactive oxygen species (ROS) and the membrane targeting of AMPs (KRWWKWIRW, a peptide screened by artificial neural networks) to synergistically enhance antibacterial activity. In addition, unlike traditional aggregation-induced quenching (ACQ) photosensitizers, aggregation-induced emission (AIE) photosensitizers exhibit stronger fluorescence emission in the aggregated state, which helps visualize antibacterial mechanisms. In vitro antibacterial experiments show that the preparation has a good killing effect on both Gram-positive bacteria (G+) and Gram-negative bacteria (Ge). The ability to induce bacterial aggregation enhances the light-activated antibacterial activity against Ge bacteria. Notably, it showed significant effectiveness in destroying MRSA biofilms. It also has significant therapeutic effects on wound infections in mice.
The excellent fluorescence properties of the fluorophore made it possible to visualize the interaction pattern between TPICysHHC10 and bacteria. In addition, the excellent singlet oxygen-generating ability of the fluorophore enhances the photodynamic antibacterial activity of TPI-CysHHC10. On the other hand, the destructive function of AMPs on bacterial outer membranes plays a crucial role in the antibacterial activity of TPI-CysHHC10 against Ge bacteria.
The aggregation-inducing effect of TPI-CysHHC10 on Ge bacteria contributes to the efficient utilization of ROS generated by the conjugates encapsulated within bacterial aggregates. This local effect of ROS in a small range enhances the photodynamic antibacterial effect of TPICysHHC10. While killing planktonic bacteria, TPI-CysHHC10 also showed good results in inhibiting the formation of MRSA biofilms and destroying mature MRSA biofilms. This multifunctional antimicrobial agent has great potential to address the challenges posed by bacterial biofilm-related infections and drug-resistant bacteria.
Fig. 2 The design mechanism of TPI-CysHHC10.1
In summary, the researchers proposed a new strategy to develop antibacterial conjugates by combining PS with AMP, thereby generating a new class of broad-spectrum antibacterial drugs. In the treatment of mrsa infected wounds, TPI-CysHHC10 showed excellent photodynamic antibacterial effect and good biocompatibility. Conjugation of antimicrobial peptides with membrane-disrupting properties to photosensitizers is a novel and effective strategy for treating bacterial infections. This multifunctional antimicrobial agent has great potential to address the challenges posed by bacterial biofilm-related infections and drug-resistant bacteria. Further research and development of this drug may lead to substantial advances in the field of bacterial infection treatment.
With our well-established DrugLnk™ and antibody engineering platforms, Creative Biolabs is dedicated to offering the best services to promote your antibody-antibiotic conjugate development. Our services include but are not limited to:
- Antibody Discovery Services for Bacterial Infection
- Linker Design and Synthesis
- Antibiotic Synthesis
- Antibody-Antibiotic Conjugate
- AAC In Vitro Analysis
- AAC In Vivo Analysis
Please contact us for more information and a detailed quote.
Reference:
- Fan, Duoyang, et al., “Harnessing antimicrobial peptide-coupled photosensitizer to combat drug-resistant biofilm infections through enhanced photodynamic therapy.” Acta Pharmaceutica Sinica B4 (2024): 1759-1771.