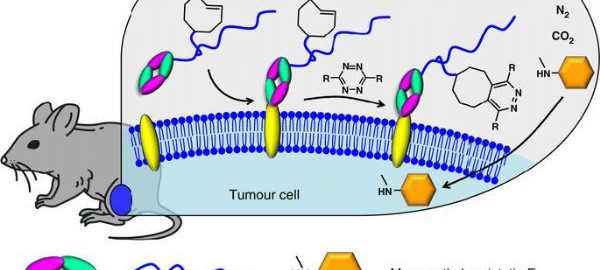
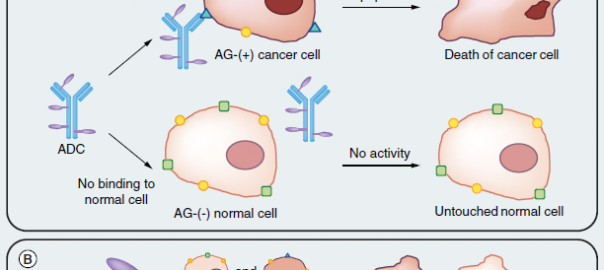
FDA Grants Priority Review for Sacituzumab Govitecan for the Treatment of Metastatic Triple-negative Breast Cancer
On July 18, Immunopharmas, a leading biopharmaceutical company in the field of antibody-drug conjugates, announced that the Biologics License Application (BLA) of its sacituzumab govitecan for the treatment of metastatic triple-negative breastRead More…