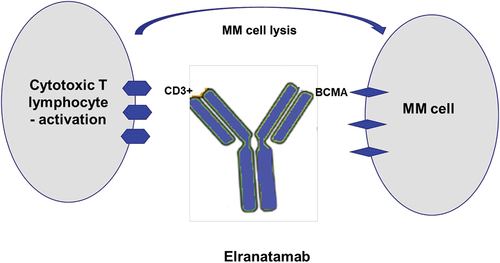
FDA-Granted Priority Review for Pfizer’s Anti-CD3/BCMA Bispecific Antibody Elranatamab
Pfizer announced that the U.S. Food and Drug Administration (FDA) has granted priority review status to its biologics license application (BLA) for elranatamab, an anti-CD3/BCMA bispecific antibody (bsAb), with a decision onRead More…