Pfizer announced that the U.S. Food and Drug Administration (FDA) has granted priority review status to its biologics license application (BLA) for elranatamab, an anti-CD3/BCMA bispecific antibody (bsAb), with a decision on the application expected from the FDA in 2023. This also signals that elranatamab is expected to be the second anti-CD3/BCMA bispecific antibody approved for marketing worldwide, following Johnson & Johnson/Genmab’s teclistamab. Meanwhile, the European Medicines Agency (EMA) has also accepted the marketing authorization application (MAA) for elranatamab.
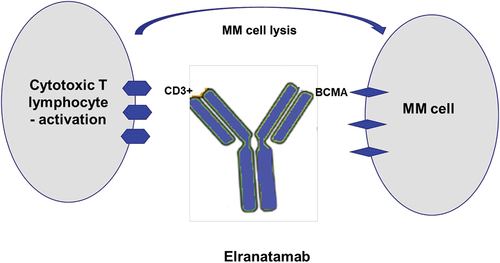
Fig. 1 Mechanism of action of Elranatamab. (Grosicki, 2023)
Elranatamab is designed to bind BCMA, which is highly expressed on the surface of multiple myeloma (MM) cells, and the CD3 receptor on the surface of T cells to activate T cells to kill myeloma cells. Elranatamab’s BLA and MAA are primarily based on data from MagnetisMM-3 (NCT04649359) cohort A (BCMA-naïve, n=123), an ongoing open, multicenter, single-arm phase II clinical study designed to evaluate the safety and efficacy of elranatamab monotherapy in patients with relapsed refractory multiple myeloma (RRMM) enrolled in patients previously treated with at least three classes of therapies including proteasome inhibitors, immunomodulators, and anti-CD38 monoclonal antibodies.
In the MagnetisMM-3 study, patients received weekly subcutaneous injections of elranatamab (76mg, QW) in 28-day cycles. Patients who receive 6 or more cycles and achieve partial remission or better remission for at least 2 months may adjust the dosing interval to once every two weeks. At a median follow-up of 10.4 months, patients receiving elranatamab as their first BCMA-targeted therapy achieved a high objective remission rate of 61% and a very good partial remission rate of 55%. The MagnetisMM-3 results also demonstrated a manageable safety profile for elranatamab.
In November 2022, elranatamab was granted Breakthrough Therapy Designation by the FDA. In addition, elranatamab has been granted orphan drug designation by the FDA and EMA for the treatment of MM, as well as Fast Track Designation (FTD) and Programs In Medical Education (PRIME) program, respectively. The UK Medicines and Healthcare products Regulatory Agency (MHRA) has also granted innovative drug designation to elranatamab. Elranatamab has been included by the FDA in the ORBIS program, a framework for simultaneous submission and review of oncology products in multiple countries, which may expedite approval in certain countries/regions outside the US.
In recent years, with the continuous advancement of biopharmaceutical technology, there has been a boom in the research of bsAb, and the successful launch of several bsAb products like elranatamab in recent years has pushed the enthusiasm of this field to the forefront again.
Compared with monoclonal antibodies, bispecific antibodies can directly mediate tumor killing by immune cells, enhance activation of immune cells, stimulate stronger endocytosis, and thus have greater specificity, targeting, and reduced off-target toxicity. In a review published in Nature in 2020, Professor Raymond Deshaies, a member of the National Academy of Sciences, said that bsAbs appear to be leading the “fourth revolution in the pharmaceutical industry”. Perhaps in the future, these small molecules will be able to better solve therapeutic problems that monoclonal antibodies cannot, providing patients with greater clinical benefits.
Reference
1. Grosicki, Sebastian, Martyna Bednarczyk, and Karolina Kociszewska. “Elranatamab: a new promising BispAb in multiple myeloma treatment.” Expert Review of Anticancer Therapy 23.8 (2023): 775-782.
