Bispecific antibody (bsAb) refers to a molecule that can bind to two different antigens or to two different epitopes of the same antigen. The concept of bsAbs was first proposed by Nisonoff and his collaborators in 1960 and published in Science. In 1975, the emergence of hybridoma technology promoted the development of bsAb technology. Since then, Milstein et al. developed the first bsAb with a complete immunoglobulin G (IgG) structure through quadroma technology in 1983.
In 2009, Catumaxomab (Removab), the first bsAb drug, was launched to control EpCAM-positive malignant ascites. However, problems still existed, including immunogenicity, stability, solubility, and manufacturability. The drug was delisted in 2017 due to commercial reasons. In 2014, Blinatumomab (Blincyto, anti-CD19×anti-CD3) developed by Amgen became the second bsAb drug on the market for the treatment of acute lymphoblastic leukemia. The third is Emicizumab (Hemlibra) launched by Roche in 2017 for routine prevention of frequent bleeding in adult and pediatric patients with hemophilia A with factor VIII inhibitors.
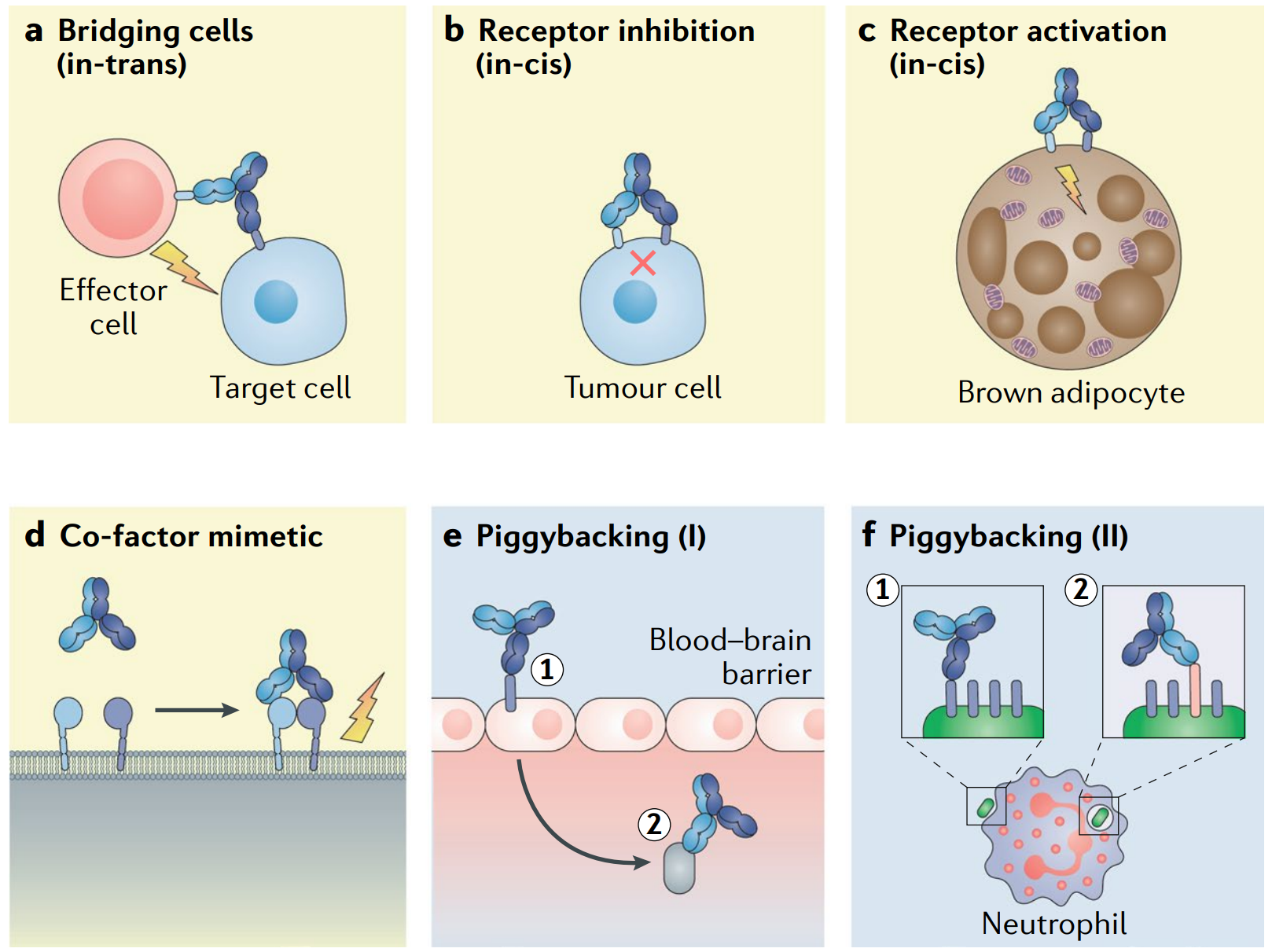
Fig. 5 Examples of obligate mechanisms of action of bsAbs. (Labrijn, 2019)
New Function of BsAb: 1+1> 2
With the success of monospecific antibody (msAb) drugs represented by PD-1/PD-L1, new bio-macromolecule drugs have been rising stars for both researchers and investors. As an upgraded version of msAb drugs, and bsAbs represent the forefront of antibody drugs, grabbing much attention. According to statistics, as of March 2019, there are about 85 bsAb projects in the clinical stage. As of September 2019, the number of projects has exceeded 110, and by June 2020, it has exceeded 160. The number continues to increase, which means that the number of bsAb projects that enter the clinic each year will maintain rapid growth in the future. This rapid development raises a question: why is bsAb so favored?
1.1 BsAb vs. Combination of MsAbs
When it comes to bsAbs, most people may first want to know the difference between bsAb and the combination of two msAbs. The latter utilizes the correlation of the target of each msAb in the process of disease regulation and synergy to enhance curative effect or reduce toxic and side effects, while bsAb also acts on two targets, so referring to target, the two have similarities. If the activity or function of the bsAb can be obtained by combining its parental antibody, this type is also called combinatorial bsAbs. On the other hand, because the ratio of the two antibody binding domains of the bsAb is fixed, it is impossible to optimize and adjust the toxicity, pharmacokinetics, and pharmacodynamics of the drug by changing the dosage ratio of different antibodies like a combination drug. In addition, the technical difficulty of the bsAb in molecular design, screening optimization, and preparation technology is more challenging than that of the msAb. It is conceivable that the development of bsAb is not just a replacement for the combination of two msAbs, it has many new activities and functions that the latter cannot achieve. Therefore, the true value of it is a series of characteristics displayed under its specific concept, such as bridging, enhancing anti-tumor immune response, and specifically activating immune response.
1.2 T Cell-Tumor Cell Bridging
Most of the bsAb drugs currently under research are aimed at tumor treatment. The common strategy in this field is to use bsAb to direct effector T cells to tumor cells for specific elimination. The bsAb molecule with this function is designed to include T cell binding domain and tumor cell binding domain, which can bind to T cells and tumor cells respectively, forming a physical connection between the two, so that T cells can better perform tumor killing effect. Such bsAbs are also called T cell-engaging bsAb (bsTCE). The second marketed bsAb drug, Blinatumomab, adopts this design and has shown excellent results in the treatment of non-Hodgkin’s lymphoma (NHL) and relapsed/refractory acute lymphoblastic leukemia (ALL). At present, more than half of the bsAb projects under research are on bsTCE.
1.3 Enhance Anti-tumor Immune Response
During the immune response, the activation of T cells requires dual-signal stimulation. The first signal comes from the T cell receptor (TCR) that recognizes the major histocompatibility complex (MHC)/antigen peptide complex and transmits antigen-specific recognition signals; the second signal is from the costimulatory molecules of antigen presenting cells (APC) to enhance TCR signals. The first signal determines the specificity of T cell activation, and the costimulatory signal determines the direction of the T cell. A large number of research have confirmed that if there is no costimulatory signal provided by costimulatory molecules, T cells will enter an unresponsive state or immune tolerance, and even cause programmed cell death.
Immune checkpoint suppression is a mechanism for the immune system to maintain self-tolerance, prevent autoimmune responses, and minimize tissue damage by controlling the time and intensity of immune responses. Tumor cells take advantage of this to weaken the immune response by making immune checkpoint signals abnormal and form an immune escape.
Based on the above two mechanisms, bsAb can simultaneously enhance T cell costimulatory signals and blocking the T cell response inhibition signal to achieve the dual effect of inhibiting tumor cell escape while activating T cells, thereby enhancing the killing of tumor cells.
1.4 Specific Activation of Immune Response
By adjusting the two different binding domains of the bsAb and the affinity of corresponding target, the immune response can be specifically activated to improve the safety and tolerability of the drug. There are two ways to realize this strategy. One is to act on the surface antigens of two different cells, and the other is to act on two different antigens on the surface of the same cell.
PD-L1×4-1BB is an example of the former method. The affinity of the bsAb and tumor cell PD-L1 is much higher than that of T cell 4-1BB, so the bsAb will preferentially bind to PD-L1 on the tumor surface. By blocking the immune escape pathway while achieving the aggregation of 4-1BB molecules, the bsAb provides conditions for the activation of T cells, thereby effectively reducing potential off-target toxicity and improving the safety. The latter method can be explained by using CD47×CD19 as an example. Because a variety of tumor cells release a “don’t eat me” signal through a high expression of CD47 to avoid being engulfed by macrophages, blocking the CD47-SIPRα signaling pathway can inhibit the immune escape of tumor cells. However, CD47 is widely distributed in various cells of the human body, especially in red blood cells and platelets with high expression levels. Therefore, antibody drugs targeting CD47 are prone to cause severe hemolytic anemia. The CD47×CD19 bsAb is designed to target the two tumor antigens CD19 and CD47, but has low affinity to CD47 and high affinity to CD19, so that it only works on tumor cells co-expressing the two antigens, thus reducing adverse reactions such as anemia. In the study of bsAbs, it was found that when acting on two targets on the same cell surface, combining with the first target or receptor will significantly increase the binding affinity to the second target. This feature is called cross-arm binding efficiency.
1.5 Structural Analogues of Cofactors
The bsAb can act like an enzyme or cofactor by mimicking the spatial structure of the enzyme-substrate binding. For example, it is designed to gather FIXa and FX through two different binding arms, thereby increasing the catalytic activity of FIXa and mimicking FVIIIa to treat hemophilia. On this basis, Emicizumab has been developed for the routine prevention of hemophilia A with FVIII factor inhibitors.
1.6 Piggyback Approaches
The above-mentioned functions of bsAbs all rely on the simultaneous binding of two different binding domains to the corresponding target, while some other functions are achieved by binding to two different targets one after the other. For example, by using the first specificity as the transportation of the second, also called hijacking approaches, antibodies are able to enter some restricted areas, such as the blood-brain barrier. In the preclinical animal study of Alzheimer’s disease, it was found that the bsAb containing the transferrin receptor (TfR) binding domain can pass through the blood-brain barrier through the transcellular effect of transferrin by binding to TfR, thereby increasing the brain transport of another binding domain.
The New Difficulty of bsAbs: 2≠1+1
In recent years, research on bsAb drugs is in full swing. The participating pharmaceutical companies and biotechnology companies have developed dozens of anti-biological technology platforms. Although nominally bsAbs are formed by the fusion of two different monoclonal antibodies, in fact, the development of bsAbs is much more complicated than that of msAbs, which involves functionality, affinity, and potency (antigen binding sites), antigen characteristics, antibody size, Fc structure, chain-related issues, etc., making the barriers to the bsAb development quite high.
2.1 Functionality
Any bsAb must have its specific functionality. The design is combining antibody fragments with different specificities. However, after the antibody fragments are reconstructed, their functionality may be different from the parent antibody. . In the screening of bsAbs, it is found that the characteristics of parent antibodies alone cannot predict the most effective bsAb molecule. Therefore, the final optimized bsAb is often screened from a large number of antibody combinatorial libraries based on experience.
2.2 Affinity
Antigen and antibody will show different degrees of affinity when they specifically bind, but when constructing bsAbs, it is not that the stronger the affinity, the better. In the study of bsTCE targeting the T cell antigen CD3, it was found that the low-affinity CD3 binding arm is more conducive to the distribution of bsTCE in tumors, and avoids rapid blood clearance mediated by CD3 and in the spleen, lymph nodes and other T cell-containing enriched in the organization. The function of specifically activating the immune response described above is also realized based on the difference in the affinity between the two different binding domains of the bsAb and the respective targets.
2.3 Potency
The design of bsAb titer is also a key factor affecting its activity. In the study of bcTCE, it is found that usually only a monovalent CD3 binding arm can achieve tumor-dependent T cell activation function, and can avoid antigen modulation or cytokine release caused by cross-linking of CD3 molecules on the surface of T cells. However, the number of antibody valences targeting tumor-associated antigens (TAA) needs to be determined according to the characteristics of the TAA.
2.4 Antigenic Characteristics
The bsAb and the target antigen can only achieve its function after forming a large number of contact points. Therefore, the design of the bsAb also needs to adapt to the characteristics of the antigen. Related studies have found that the activity of bsTCE is correlated with the expression level of antigen. Although this view has not yet become a generally accepted conclusion, the fact is that bsTCE can produce cytotoxicity only after the antigen expression level reaches a certain threshold. And there are differences in the thresholds of different antigens. In addition, factors such as the size of the antigen, the specificity of the tumor, the size and conformation of the extracellular end, the distance between the epitope and the cell membrane surface, and the fluidity of the cell membrane where the antigen is located will affect the function of bsTCE.
2.5 Antibody Size
Compared with bsAbs with large molecular weight, bsAbs with small molecular weight are more likely to penetrate into tumor tissues. In the process of bsTCE development, bsTCE needs to pull T cells and target cells to a specific distance to form an immune synapse, and then produce T cell-mediated cytotoxicity. Therefore, in addition to the factor of the distance between the epitope and the cell membrane surface, the size of the bsAb also affects the activation of T cells. But on the other hand, small-sized bsAbs are easier to be eliminated by the kidneys than natural antibodies, and have a shorter half-life in the body. Therefore, the size of the antibody should be designed according to the intended indication, in terms of penetration capacity, half-life, immune activation, etc.
2.6 Fc Structure
The Fc (fragment crystallizable) of the antibody can bind to the receptor FcRn, reduce the degradation of the antibody in the cell, extend the half-life of the antibody, and can also bind to the receptor on the immune cell to stimulate antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and antibody-dependent cellular phagocytosis (ADCP). In addition, Fc fragments can also improve the solubility and stability of antibodies. However, the bsAb containing the Fc fragment is usually large in molecular weight, which results in the reduction of the penetration of tumor tissues, and the Fc fragment is also considered to be immunogenic, which activates T cells through the binding of Fc and FcγR, and cause a tumor-independent immune response. Therefore, in the development of bsAbs, whether to retain the Fc fragment or to engineer it is also a key consideration.
2.7 Chains
The natural antibody structure is composed of two heavy chains (H chains) and two light chains (L chains). In a msAb, the amino acid composition of the two heavy chains and the two light chains is exactly the same, and there is only one structure after the combination. In contrast, the initial bsAb development strategy was achieved by co-expression of two different heavy chains and two different light chains, which resulted in 10 possible H2L2 structural combinations, and only one of them is the target product. Therefore, obtaining target functional bsAbs from 10 possible H2L2 recombination mixtures has become one of the initial challenges in the development of bsAbs. This is usually referred to as mismatch.
Some other factors, such as solubility, stability, half-life, immunogenicity, are all external manifestations of the above problems, and will not be discussed in detail here.
Reference
1. Labrijn, Aran F., et al. “Bispecific antibodies: a mechanistic review of the pipeline.” Nature reviews Drug discovery 18.8 (2019): 585-608.
