Distinctive BsAb Platform
The problem of chain mismatches will reduce the yield of the bsAb, increase the difficulty of purification, and increase the production cost. Therefore, obtaining a correctly assembled bsAb has become a key issue. In the past few decades, many strategies have been developed to improve the homogeneity of the product and the yield of the target molecule, which have become the basis of various bsAb technology platforms. These technology platforms have their own unique molecular construction formats, and there are currently more than one hundred types, which can be divided into fragment-based bsAb and full-length bsAbs according to their design features or functional characteristics.
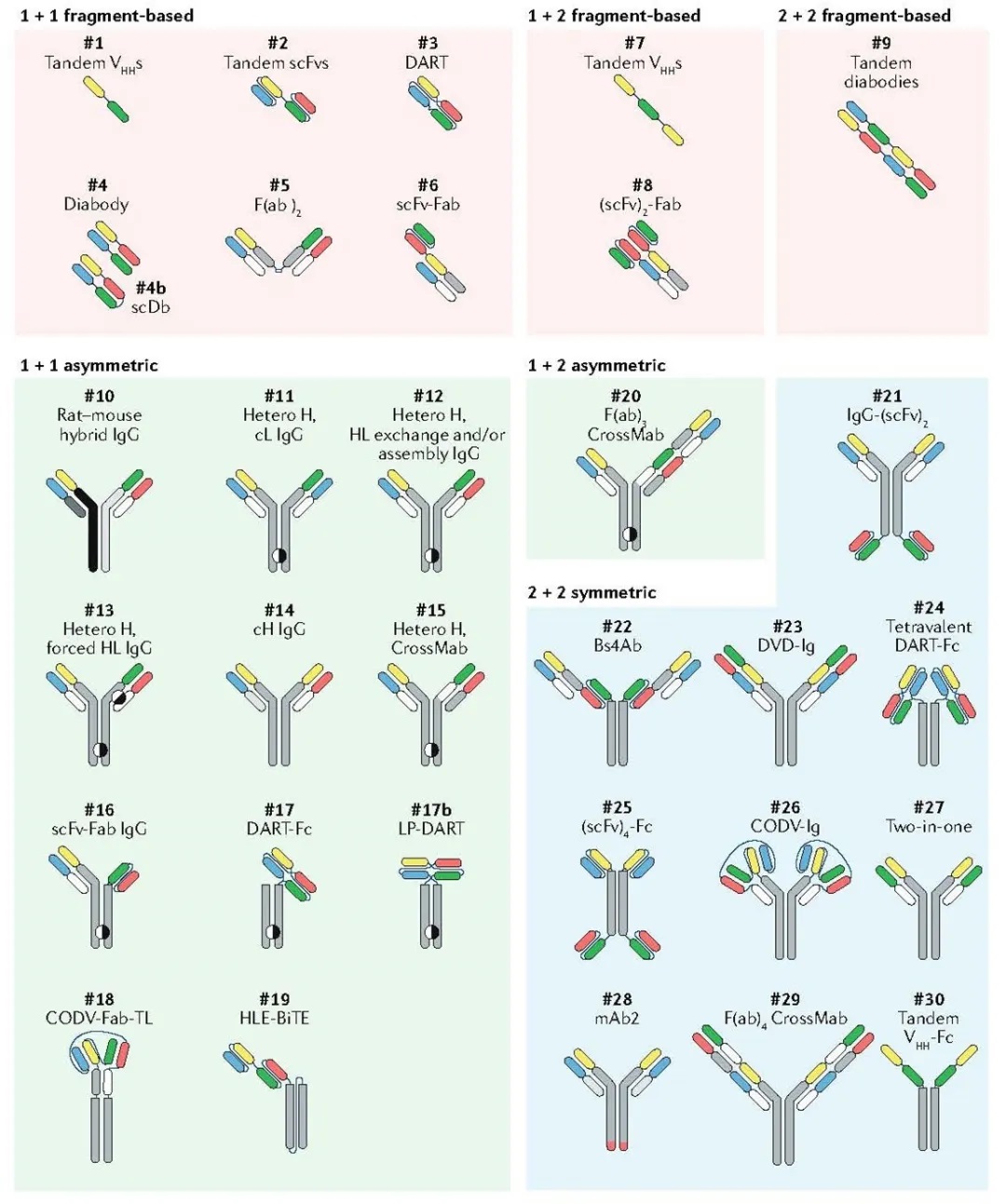
Fig. 2 A selection of bsAb formats. (Labrijn, 2019)
3.1 Fragment-based BsAb
Fragment-based bsAbs simply combine multiple antibody fragments in one molecule and do not contain an Fc region to avoid chain-related problems. The structure of antibody fragments of this type of bsAb often uses antigen-binding fragments (Fab), nanobodies (VHH), single-chain antibodies (scFv), etc., as modules in series, combining antibody fragments of different specificities into 1+1, 1+2,2+2, and even 1+3, 3+3 structures according to their titers. This kind of bsAb does not have a complicated structure, so the production process is relatively simple, the yield is high, the cost is low, and the absence of Fc fragments can give it lower immunogenicity, while the smaller molecular weight gives higher tumor tissue permeability. On the other hand, the lack of Fc fragments also makes them lose some of the advantages of intact antibodies. Therefore, the fragment-based bsAb has the disadvantage of relatively short half-life, and may also cause stability and aggregation problems. The currently commercialized technology platforms such as BiTE, DART, TandAb, and Bi-Nanobody are all based on fragments.
BiTE (bispecific T cell engager) is a tandem single-chain antibody technology developed by Micromet (purchased by Amgen in 2012). It consists of a scFv that binds T cell antigen (such as CD3) and a scFv that binds TAA through a peptide linker. BiTE bsAb can simultaneously bind to T cells and tumor cells, recruit T cells to the surface of tumor cells, thereby activating T cells to kill tumors. BiTE technology overcomes the production problems of poor stability, low expression, and low solubility of scFv, and has successfully achieved commercialization. The representative drug is Blinatumomab.
DART (dual affinity retargeting) is a new generation of bsAb construction technology jointly developed by MacroGenics and Servier. DART bsAb is a heterodimeric antibody formed by the combination of two polypeptide chains, with two unique antigen binding sites. Its structure is formed by connecting the VH and VL sequences of one antibody variable region with the VL and VH sequences of another antibody variable region, respectively. In addition, cysteine is introduced at the C-terminus of the two polypeptide chains to form interchain disulfide bonds, which improves the stability of the product. Different from the above-mentioned BiTE technology connected by a peptide linker, the design of DART can mimic the natural interactions within the IgG molecule, and can maintain efficacy in both in vitro and in vivo administration, and the scale of aggregation in production is lower.
TandAb (Tandem diabody) is a tetravalent bsAb developed by Affimed, which has two binding sites for each of the two antigens. Its structure is Fv1-Fv2-Fv2-Fv1, which is a homodimer molecule formed by the reverse pairing of two polypeptide chains. The relative molecular weight of TandAb is about 105kDa, which exceeds the threshold of renal clearance. Compared with small molecule antibodies, the half-life can be extended to about 23h. In addition, the protein has no glycosylation modification, so the product is more uniform, and the immunogenicity is lower.
Bi-Nanobody was developed by Ablynx (purchased by Sanofi in 2018). It is a single domain antibody derived from the variable region fragment VHH of a heavy chain antibody that lacks light chains in camels and llamas. The main advantages of it are small molecule, high stability, easy to humanize, easy to connect. Small molecule makes it easy to penetrate deeper tissues and target some epitopes that are difficult to reach by normal IgG antibodies. However, nanobodies lacking Fc have a shorter circulating half-life in the body, and the half-life can be extended to 2 to 3 weeks by fusing human serum albumin or albumin functional regions.
3.2 Full-length BsAb
Full-length bsAb can be further divided into symmetric and asymmetric bsAbs. The symmetric bsAb is the fusion of two different specific antigen binding domains in a single polypeptide chain or a single set of paired light and heavy chains, while retaining the Fc region. It is composed of a pair of identical polypeptide chains or paired light and heavy chains. Although they are relatively close to natural antibodies, they are still different from the latter in molecular size and structure. This type of bsAb combines the conventional antibody molecular structure with antibody fragments, which tends to increase the volume of the antibody and change the performance of the antibody under the natural structure (such as stability, and solubility), which may affect the physical and chemical properties and pharmacokinetic properties of the bsAb. Most symmetrical bsAbs use a 4-valent 2+2 structure, but in this structure, if the antigen binding site is too close, it may affect the efficiency of simultaneous binding with the two targets. Technology platforms such as DVD-Ig and Two-in-one are all symmetrical models.
The DVD-Ig technology was developed by Abbott. Its structure is to connect the VL and VH domains of another antibody at the N-terminus of the normal IgG antibody light chain and heavy chain, respectively, to produce a binding site with two binding sites for each antigen. The DVD-Ig bsAb has the same Fc region as the normal monoclonal antibody, and can be produced using the existing general antibody technology.
Two-in-one technology was first proposed by Genentech, which, by engineering the variable region and phage display technology, obtains bispecific antibodies that recognize two different targets, and is also called DAF antibody (Dual Action Fab, DAF). The Two-in-one bsAb has the structure of a normal IgG antibody, showing a high level of stability, and easiness of industrial production. It has outstanding advantages in downstream production processes, formulation development, and in vivo pharmacokinetics. The technical difficulty lies in the early stage of engineering process.
The number of asymmetric patterns is greater than that of symmetric patterns, and a considerable part of them uses a structure similar to natural antibodies in order to retain the advantages of natural antibodies as much as possible, but it will also destroy the symmetrical structure of H2L2 and causes the chain-related problems mentioned above. Therefore, most asymmetric models are based on the strategy of promoting heavy chain heterodimerization and correct pairing of light and heavy chains when co-expressing four polypeptide chains or three polypeptide chains (such as using common light chain technology).
The heavy chain heterodimerization of the bsAb can combine two different but complementary heavy chains through the engineering of the Fc region to form the correct pairing between the heavy chains. Commonly used technology includes Knobs-into-holes, KiH and ART-Ig. The heavy chain heterodimerization technology can reduce the structural combination of H2L2 from the original 10 structures to 4.
KiH technology mutates a smaller threonine (T) in the CH3 region of one of the heavy chains of the bsAb to a larger tyrosine (Y), forming a prominent Knob structure (T366Y), and at the same time mutates a larger tyrosine (Y) residue in the CH3 region of the other heavy chain into a smaller threonine (T) to form a recessed hole structure (Y407T). The steric hindrance effect of the KiH structure leads to the correct assembly between two different heavy chains. The KiH structure can make the correct assembly rate reach 90%-95%, which meets the requirements of large-scale production. This technology was developed by Genentech in 1997, and the current patent has expired, so it has become one of the most commonly used technologies in the development of bsAb, and it is often used as a basic platform combined with other technologies to form a new platform.
ART-Ig is a representative technology based on electrostatic manipulation and interaction, developed by Chugai, a subsidiary of Roche. This technique substitutes negatively charged aspartic acid or glutamic acid for a residue in the CH3 region of one heavy chain, and substitutes a positively charged lysine for a residue in the CH3 region of the other heavy chain. Different heavy chains promote the formation of heavy chain heterodimers through the attraction of opposite charges, while the same heavy chains inhibit the formation of homodimers through the mutual repulsion of same charges.
Although the Fc region of the bsAb can be engineered to promote correct heavy chain heterodimerization, the use of two different light chains will still result in the production of 4 different combinations, and only one of them is the target bsAb. Therefore, common light chain technology and CrossMab technology have been developed to promote the correct pairing of light chain and heavy chain.
The bsAb designed by the common light chain technology has only one light chain, but it can be paired with two different heavy chains, which directly eliminates the problem of mismatch between the light chain and the heavy chain. This strategy is derived from the findings of phage display screening studies against multiple antigens, in which antibodies usually share the same VL domain. Although the common light chain is more convenient in design and expression, and it simplifies the antibody engineering and purification process in industrial production, but it limits the flexibility of antibody screening and construction.
The CrossMab technology developed by Roche is based on the KiH structure, swapping CL and CH1 in one Fab domain of the bsAb, while the other Fab domain remains unchanged. The structure of CL and CH1 is similar, so the interchange position has little effect on the function of the antibody; the light chain of the modified Fab domain is less possible to mismatch with the heavy chain of another normal Fab domain, thus realizing the correct pairing. Roche has developed representative products, RG7221 and RG7716, with CrossMab technology, both of which are anti-Ang-2/VEGF bsAbs.
New BsAb Drugs Are Ready to Go
So far, three bsAb drugs have been approved for marketing worldwide—Catumaxomab, Blinatumomab and Emicizumab. Blinatumomab and Emicizumab occupy the entire current bsAb market, and their global sales in 2019 reached US$312 million and US$1.52 billion, respectively. Emicizumab has previously obtained the breakthrough drug, the orphan drug and priority qualifications, and it is predicted that there is still a lot of room for growth in future sales.
In addition to the above three drugs, Janssen has submitted a marketing application for the EGFR×cMET bsAb, Amivantamab, to the United States Food and Drug Administration (FDA) in December 2020, and there are many other bsAb projects under development worldwide. According to statistics, as of June 2020, there are about 165 bsAbs in the clinical development stage and about 270 in the pre-clinical development stage worldwide, and the number of new projects has maintained rapid growth. Most of the bsAb projects entering the clinical development stage are still in the early clinical research phase (67% are in clinical phase I, 28% are in clinical phase II), and only 9 are in clinical phase III. In terms of indications, about 84% are used to treat cancer, and some are being developed to treat autoimmune system diseases. In terms of the design strategy, nearly half bsAbs kill tumor cells by activating T cells through CD3 or activating NK cells through CD16; another common strategy is to specifically limit the functioning conditions of the bsAb to only make it only kill tumor cells, thereby reducing damage to normal cells or tissues.
Prospect: The Coexistence of Opportunity and Competition
As a cutting-edge technology, the challenges faced by the development of bsAb drugs far exceed those of msAbs. The paper published by Dr. Siwei Nie and other experts in Antibody Therapeutics has summarized the three principles for designing and identifying potential new bsAbs or polyspecific antibodies: (1) the molecular structure design of bsAbs should be driven by unique biological mechanisms; (2) the construction mode of bsAbs should be matched with biological mechanism; (3) an ideal bsAb that can enter clinical research should be selected. The paper also mentions and six elements for bsAb development—ideal clinical efficacy, safety, pharmacokinetics/pharmacodynamics, physical and chemical properties, clinical and commercial production, and immunogenicity.
Nevertheless, many bsAb projects have not fully complied with them or have chosen to ignore them in the development stage. The reason may be that the biological function indicators (such as effectiveness, safety, pharmacodynamics/pharmacodynamics and immunogenicity) and developability indicators (such as expression level, solubility, stability, viscosity) are often not completely related, and sometimes even contradictory. Therefore, how to fully balance the characteristics of various elements and design a bsAb molecule that can not only have good efficacy, but also meet the production and commercialization standards, is the basis of the clinical development.
Focusing on tumor diseases is another feature of the current bsAb development, and recruiting the tumor-killing cells through T cells is the main strategy to achieve this goal. Although this is currently one of the most efficient bsAb development approaches, it also limits the range of options for target combinations. Researchers are also constantly exploring the therapeutic potential of bsAbs in other disease fields, such as viral and bacterial infections, osteoporosis, diabetes, and autoimmune deficiency. Through animal experiments, it has been found that bsAbs can improve treatment of Ebola virus infection and the penetration rate of the blood-brain barrier.
After years of accumulation, bsAb technology has made considerable progress. A number of pharmaceutical companies and biotechnology companies have developed dozens of technology platforms based on their own technical characteristics. These technology platforms have their own advantages and disadvantages, and it is still worth continuous study and optimization, thus developing a platform with high druggability, production process feasibility and scalability, contributing to the further development of the bsAbs. As a cutting-edge technology, it’s believed that the bsAb technology can be developed into more and more effective therapeutic drugs, and bring more positive news.
Reference
1. Labrijn, Aran F., et al. “Bispecific antibodies: a mechanistic review of the pipeline.” Nature reviews Drug discovery 18.8 (2019): 585-608.
