In 2018, Loxo Oncology’s “unlimited cancer” targeted therapy Vitrakvi was approved by the U.S. Food and Drug Administration (FDA), becoming the first targeted therapy for patients with neurotrophic receptor tyrosine kinase (NTRK) gene fusion variant solid tumors. Loxo, on the other hand, had begun to develop a new generation of tyrosine receptor kinase (TRK) inhibitors before Vitrakvi was approved. Although Vitrakvi, as a TRK inhibitor, achieves a remission rate of 75 percent in patients with solid tumors, over time, tumors acquire gene mutations that are resistant to TRK inhibitors. Therefore, it is urgent to develop a new generation of inhibitors that can be effective against these tumors.
On April 2, Bayer announced the results of a new generation of TRK inhibitor LOXO-195 developed by Loxo in early clinical trials. Bayer acquired LOXO-195 ‘s exclusive R&D interest after Loxo was acquired by Lilly. LOXO-195 can inhibit the activity of TRK protein which is already resistant to existing TRK inhibitors. The results showed that LOXO-195 achieved an objective remission rate of 45 percent (ORR) in 20 patients with solid tumors who had been treated with a TRK inhibitor and were resistant to it. Although this study is still in its early clinical stages, it suggests that LOXO-195 may help address the unfinished medical needs of patients with solid tumors who are resistant to existing TRK inhibitors.
Poly ADP ribose polymerase (PAPR) inhibitors are a class of drugs that treat tumors that carry BRCA gene mutations or homologous recombination DNA repair mechanisms through a “synthetic lethal” mechanism. They have shown excellent efficacy in the treatment of ovarian and breast cancer. Recent studies have shown that such drugs can also play a role in cancer types other than breast cancer. In February 2019, Lynparza, developed by AstraZeneca and MSD, served as a maintenance therapy and reached the main endpoint in a phase 3 clinical trial for the treatment of pancreatic cancer. At the American Association for Cancer Research (AACR) on April 3, Clovis’s PARP inhibitor Rubraca also obtained positive data in a phase II clinical trial of pancreatic cancer.
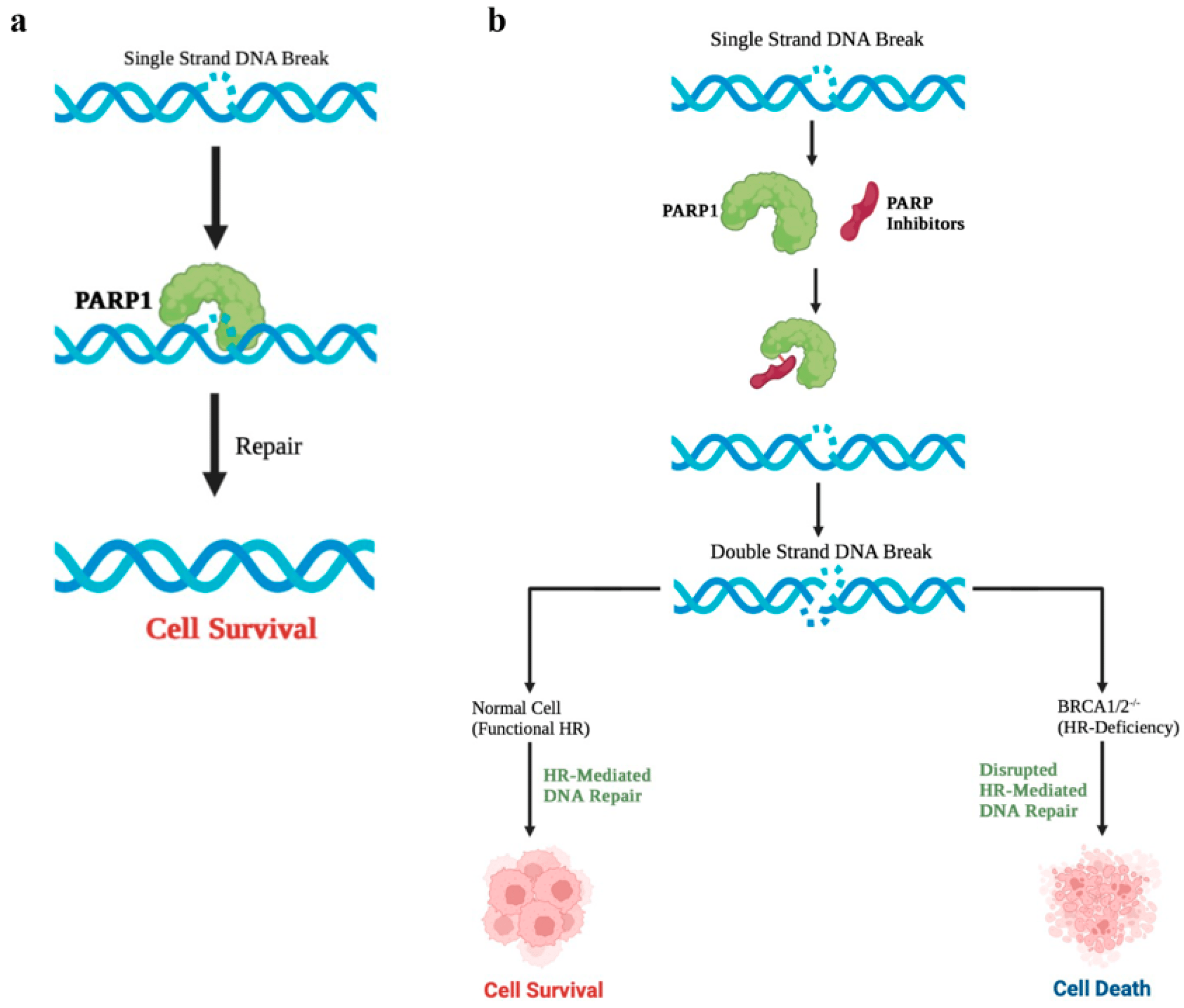
Figure 1. Mechanism of PARP inhibitors. (Dilmac, 2023)
In the single-arm phase II trial of 42 registered patients with advanced pancreatic cancer, Rubraca was used as a first-line maintenance therapy for patients who had received platinum-based chemotherapy and whose disease had not progressed over the past four months. These patients carry mutations in the pathogenic reproductive system or somatic BRCA1, BRCA2 or PALB2 genes.
Mid-term data analysis showed that the median progression-free survival (PFS) was 9.1 months in 19 patients who could be evaluated. Of the 19 patients, one achieved complete remission and six achieved partial remission. These patients carried BRCA2 gene mutation (n ≤ 4), PALB2 gene mutation (n ≤ 2) and somatic BRCA2 gene mutation (n ≤ 1).
These preliminary data, combined with Lynparza’s phase 3 data, suggest that PARP inhibitors may provide a new treatment for advanced pancreatic cancer. About 5 ≤ 8% of patients with pancreatic cancer carry BRCA1, BRCA2 or PALB2 pathogenic mutations.
Bispecific antibody and polyspecific antibody are becoming a new mode of cancer treatment. At the AACR conference, Inovio presented preclinical data on the company’s innovative DNA-encoded bispecific T-cell engager (dBiTE). One end of the bispecific T cell engager (BiTE) can bind to the specific antigen on the tumor surface, while the other end can bind to the CD3 receptor on the T cell surface to recruit T cells around the tumor and play a role in killing tumor cells.
One of the challenges faced by BiTE-type proteins is the low stability of these proteins, resulting in patients requiring frequent treatment. Inovio’s innovative strategy is to introduce DNA encoding BiTE protein into human cells, making human cells a “factory” for BiTE production, thereby continuing to provide BiTE protein.
Preclinical studies published by AACR have shown that dBiTE targeting human epidermal growth factor receptor 2 (HER2) protein developed by Inovio can maintain a high level of BiTE protein expression in mice after a single treatment for 4 months in mice with breast and ovarian cancer models. Moreover, dBiTE targeting HER2 can effectively stimulate the cytotoxicity of T cells to tumor cells expressing HER2, resulting in close to complete tumor clearance.
Dr. Joseph Kim, President and Chief Executive Officer of Inovio, said that based on these positive preclinical results, the company is ready to quickly push dBiTE into clinical development.
Reference
1. Dilmac, Sayra, and Bulent Ozpolat. “Mechanisms of PARP-Inhibitor-Resistance in BRCA-Mutated Breast Cancer and New Therapeutic Approaches.” Cancers 15.14 (2023): 3642.
