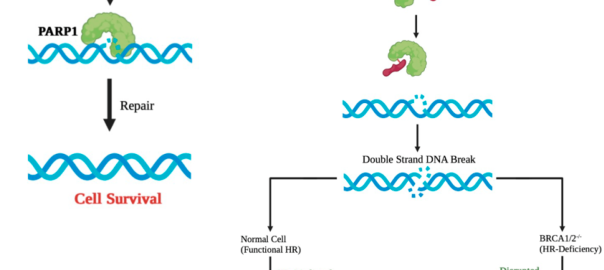
Recent Advances in Hot Areas of Bispecific Antibodies Such as PARP Inhibitors
In 2018, Loxo Oncology’s “unlimited cancer” targeted therapy Vitrakvi was approved by the U.S. Food and Drug Administration (FDA), becoming the first targeted therapy for patients with neurotrophic receptor tyrosine kinase (NTRK)Read More…