The unique advantages of CAR-T therapy in the treatment of hematological tumors have once again become a hot spot for the recent American Society of Hematology (ASH) Annual Meeting, and bispecific antibodies (BsAbs) with similar mechanisms of action also attracted people’s attention. At the ASH Annual Meeting, Regeneron and Amgen have published early clinical data on their bispecific antibodies, which are comparable to CAR-T therapy. At the same time, Bluebird, Celgene, Juno, Allogene, etc. also announced the early clinical development data of their CAR-T therapy.
CAR-T and BsAb
Chimeric Antigen Receptor T-Cell Immunotherapy (CAR-T) has a similar mechanism of action as Bispecific Antibody (BsAb) therapy, both of which can enable T cells to effectively target the tumor-associated antigen, enhancing the killing effect of T cells on tumor cells.
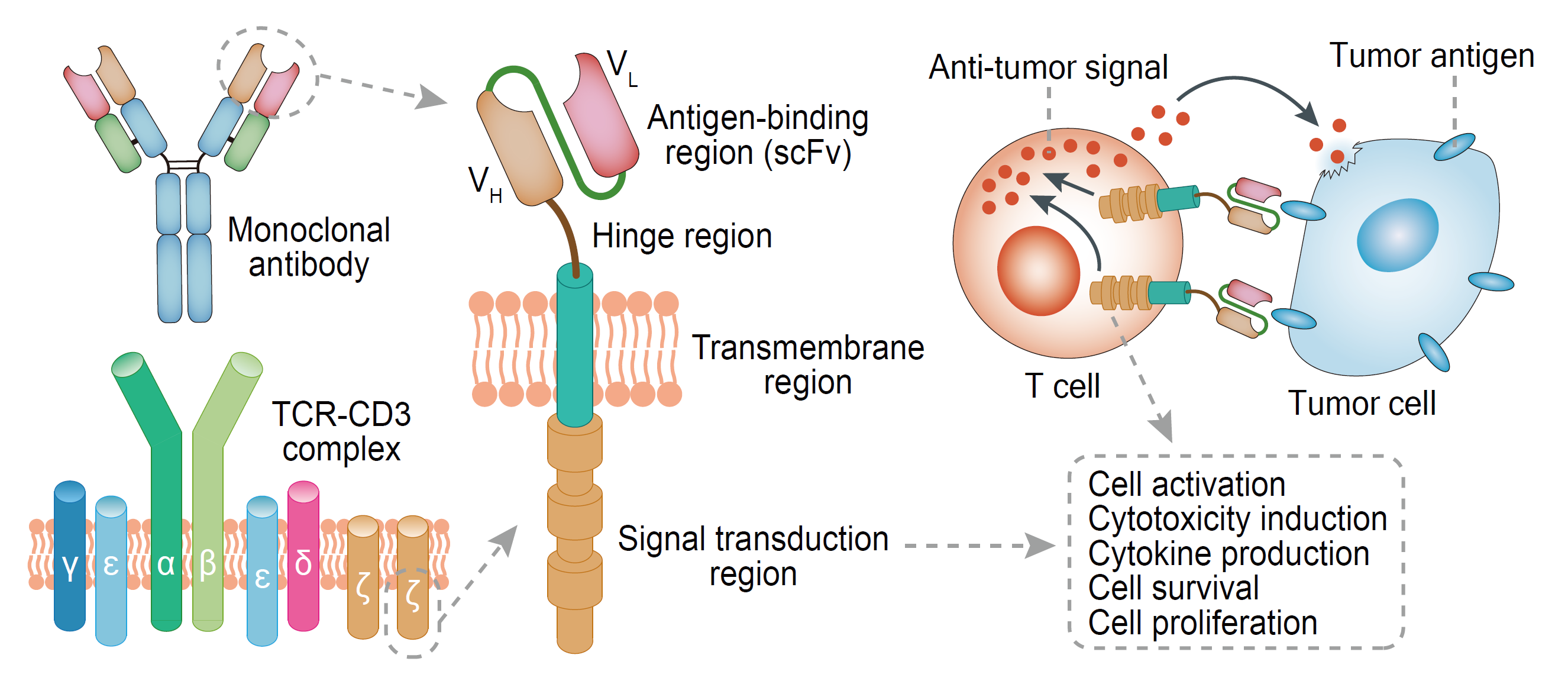
CAR-T therapy genetically engineers patient T cells in vitro, which combines a single-chain antibody (scFv) that recognizes tumor-associated antigens, a transmembrane co-stimulatory domain (such as CD28 or CD4-1BB), and a T cell activation motif (such as CD3ζ or FcεRIγ). T cells are transfected by gene transduction to form CAR-T cells and then returned to the patient to function after expanded in vitro. CAR-T cells can enhance the ability to bind to tumor cells by expressing single-chain antibodies, thereby increasing the killing ability of cancer cells.
Fig.1. CAR structure
Bispecific antibodies (BsAbs) are mainly classified into two major formats: BsAb (IgG-like) containing Fc region and BsAb (Non-IgG-like) containing no Fc region. Their two single-chain antibodies (scFv) in Fab region can bind to T cell antigens (such as CD3) and tumor cell antigens (such as EpCAM, etc.), which links the T cells and tumor cells, and initiate accurate killing of tumors by T cells. The preparation methods of BsAb include chemical coupling method, double hybridoma fusion method (Konb-in-hole, etc.) and genetic engineering.
FDA-approved CAR-T and BsAb therapies
In 2017, the FDA successively approved two CAR-T therapies for the treatment of hematoma, Kymriah and Yescarta. In 2014, the first BsAb Blincyto (blinatumomab) received FDA’s approval for the treatment of relapsed/refractory precursor B-cell acute lymphoblastic leukemia. In 2017, the FDA approved the Hemlibra (emicizumab–kxwh) developed by Roche, for the treatment of hemophilia A, which targets coagulation factors IXa and X.
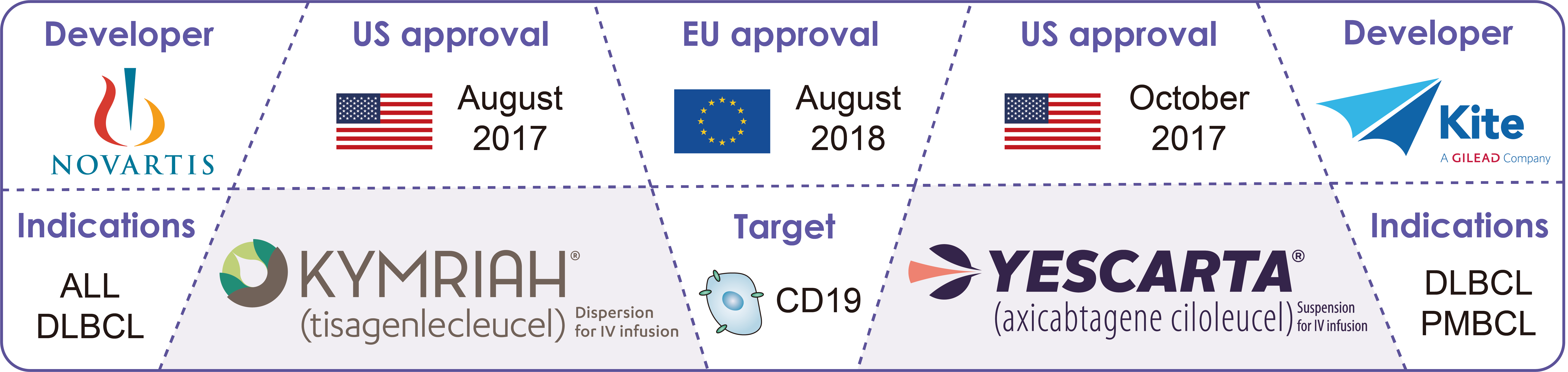 Fig.2. FDA-approved CAR-T therapies
Fig.2. FDA-approved CAR-T therapies
In addition, Catumaxomab, developed by Trion Pharmaceuticals in 2009, was approved for marketing in the European Union as the world’s first approved BsAb. It is a mouse bispecific antibody against CD3 and epithelial cell adhesion molecule (EPCAM) for the treatment of patients with malignant ascites with EPCAM-positive tumors.
There are also many CAR-T and BsAb programs in clinical trials, in which CAR-T therapies include LCAR-B38M, bb2121, CART-BCMA, JCARH125, FCARH143, bb21217, etc., and BsAb therapies include AMG330, AMG420, REGN1979, Mosunetuzumab, COVA322, Remtolumab, RG6026, etc.
Clinical data of BsAb and anti-BCMA CAR-T therapies
At the 2018 ASH Annual Meeting, Amgen once again demonstrated the clinical data of its bispecific T cell adapter (BiTE) AMG420, whicn targets BCMA and CD33, in the treatment of relapsed/refractory multiple myeloma (MM). In a phase I study of dose escalation (0.2-800 ug/day), 13 MM patients received a 400 ug/day dose of AMG420, achieving an objective response rate (ORR) of 70% (7/10) and a complete response rate(CR) of 40%, of which 6 patients had a remission period of 7.5 months.
However, AMG420 is less effective compared with the anti-BCMA CAR-T therapy. Bb2121, a CAR-T therapy targeted BCMA developed by Bluebird and Celgene, achieved an ORR of 83% in 12 patients; and JCARH125, developed by Celgene and Juno, which also targets antigen BCMA, achieved 82% (36/44) objective response rate.
In terms of safety data, cytokine release syndrome (CRS) and neurotoxin are the main side effects of CAR-T therapy. At the ASH annual meeting, some scholars combined inhibitor Ibrutinib targeting Bruton’s tyrosine kinase (BTK) and CAR-T therapy, reducing the side effects of CAR-T therapy, which can also be achieved by a reasonable clinical treatment regimen.
For BsAb, safety is still a major consideration. Two patient deaths occurred in clinical trials of AMG420, although Amgen stated that it was not related with AMG420. Prior to this, two new BsAb candidate drugs, COVA322 (Phase I: NCT02243787) and 4G7 × H22 (Phase I: NCT00014560), were discontinued due to safety and toxicity, respectively.
In addition, due to the special structure of BsAb, such as AMG420 (BiTE), it generally has a short half-life, and the constant injection to ensure efficacy becomes a burden for patients. With regard to the short-lived shortcomings of AMG420, Amgen has embarked on the development of a next-generation BiTE-AMG701 with a longer half-life in order to gain a competitive advantage over CAR-T therapy.