T-cell receptor (TCR) gene therapy is a form of cellular immunotherapy where peripheral blood T cells from cancer patients are genetically engineered ex vivo to express tumor-specific TCRs before being reintroduced into the patient’s body. Compared to other immunotherapies like immune checkpoint blockade or tumor-infiltrating lymphocyte therapy, therapeutic TCR gene transfer allows precise control over the engineered T cells’ specificity, which strictly depends on the selected TCR. Furthermore, TCR-engineered T cells constructed ex vivo permit additional genetic modifications to maximize their activity and persistence in vivo.
However, in many patients, tumor-specific TCRs represent only a small fraction of the peripheral or tumor-infiltrating TCR repertoire, complicating the identification of relevant therapeutic TCRs from patient sources. Additionally, many highly immunogenic tumor antigens, such as cancer neoantigens generated by patient-specific tumor mutations, are unique to individual patients, highlighting the need for truly personalized approaches in discovering therapeutic TCRs.
While the role of major histocompatibility complex (MHC) class I-restricted CD4+ T cells in anti-tumor immunity is well-established, recent data emphasize the importance of MHC class II-restricted CD4+ T cells in direct anti-tumor cytotoxicity and enhancing the activity of tumor-specific CD8+ T cells, crucial for tumor control and immune therapy responsiveness. Thus, there is a need for new technologies capable of high-throughput discovery of both MHC class I and class II-restricted TCRs at the individual patient level.
On April 23, 2024, Ely Porter from RootPath, and Wouter Scheper from the Netherlands Cancer Institute published a study titled “Discovery of tumor-reactive T cell receptors by massively parallel library synthesis and screening” in the journal Nature Biotechnology. This research developed a high-throughput, personalized technology for discovering T-cell receptors (TCRs), combining the assembly of large-scale synthetic TCR libraries with high-throughput genetic screening to screen and discover tumor-reactive TCRs in a single experiment, significantly reducing the time required for TCR discovery and promising to be a powerful tool for designing cellular immunotherapies.
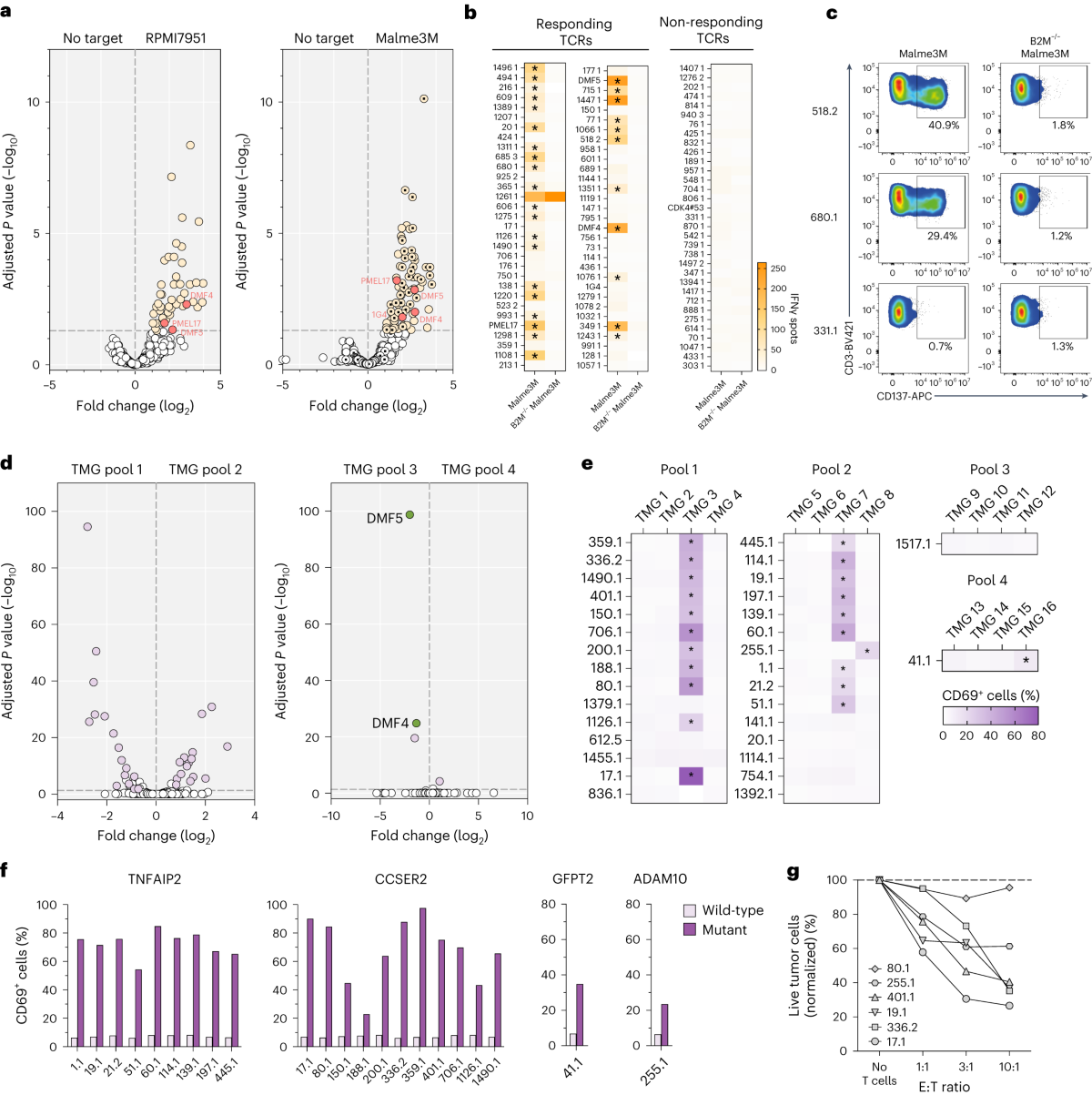
In this study, the research team developed a high-throughput, personalized TCR discovery technology that integrates the assembly of large-scale synthetic TCR libraries with high-throughput genetic screening, enabling functional analysis of specificity for thousands of individual TCRs in a single experiment. Importantly, by generating patient-derived synthetic TCR libraries, this method allows evaluation of TCR specificity independent of patient T-cell phenotypic adaptability and clonal abundance.
Using this technology, the research team screened thousands of TCRs derived from tumor-infiltrating lymphocytes (TILs) from multiple cancer patients and identified dozens of potent tumor-reactive TCRs sourced from CD4+ and CD8+ T cells, including those recognizing patient-specific neoantigens.
The researchers indicate that this method facilitates the identification of tumor antigen-specific TCRs for next-generation personalized TCR gene therapy. By employing a meticulously optimized workflow compliant with good laboratory practice (GLP), the discovery of therapeutic TCRs using this technology is expected to be achieved within 4-5 weeks (from patient tissue sampling to TCR validation), whereas discovery of existing TCRs or tumor antigens could typically take 3-5 months. Thus, the approach proposed by this study represents a potent tool for future design of cellular immunotherapies.
Creative Biolabs masters the most advanced CAR/TCR technology. With state-of-art TCR development platforms and advanced technologies, Creative Biolabs is capable of offering a broad range of TCR-T therapy development services, including TCR engineered T cell biomarker identification and selection, design, construction, and analysis.
Reference
1. Moravec, Ziva, et al. “Discovery of tumor-reactive T cell receptors by massively parallel library synthesis and screening.” Nature Biotechnology (2024): 1-9.