Cancers don’t come from anything but result from unexpected mutations in human cells with the uncontrolled proliferation of cells as the most important feature. The cells in the body undergo mutations at any time, and so do the cancer cells. But the rapid proliferation of cancer cells increases their mutation rate indirectly and during the same time, cancer cells multiply more and mutate more than body cells, contributing to different cell subtypes for the same cancer. When treated with a single drug (cells, proteins, or chemistries), only part of cancer cells can be killed, and the remaining cells become drug-resistant and continue rapid proliferation, eventually resulting in a non-effective therapy (each mutation may be a potential drug resistance).
Therefore, more attention has been paid to the combination therapy program in clinical research. At present, the popular and highly promising therapies include oncolytic virus immunotherapy strategies, immune checkpoint therapy, and the intercombination drug administration between T cell immunotherapies.
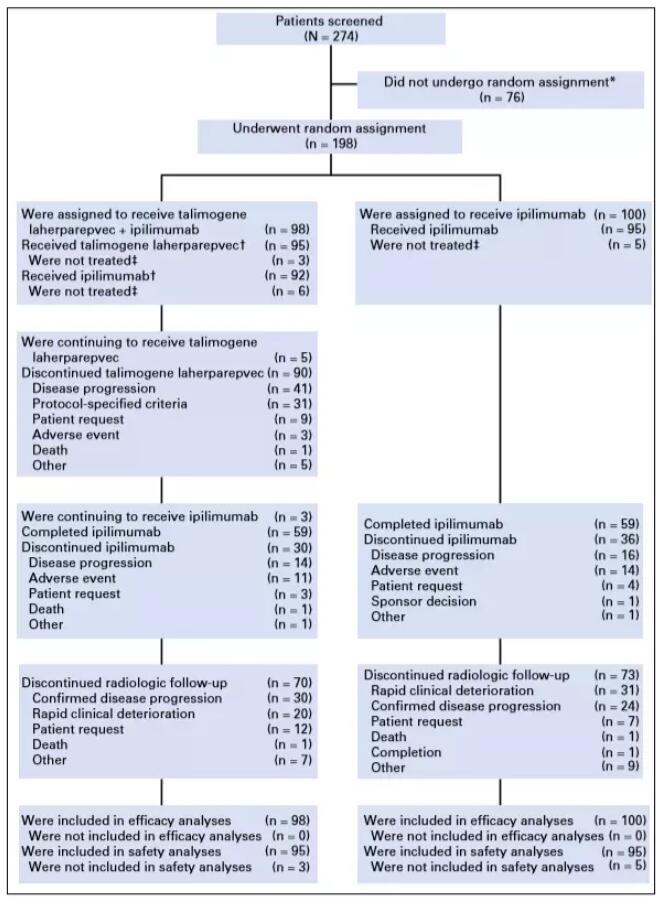
On June 10, 2018, Dr. Jason Chesney of the James Graham Brown Cancer Center at Louisville University in the United States published a report entitled “Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination with Ipilimumab VersusIpilimumab Alone in Patients with Advanced, Unresectable Melanoma” (hereinafter referred to as Paper 1). The author evaluated differences in the efficacy of the oncolytic virus T-VEC (Tailmogene laherparepcc) in combination with Ipilimumab and Ipilimumab alone in the treatment of melanoma. The results showed that combination therapy can significantly improve the treatment effect.
In September 2017, a similar research paper was published in Cell entitled Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy, which showed that the combination treatment of an oncolytic virus drug T-VEC (talimogene laherparepvec) and PD-1 antibody drug (pem-brolizumab) in melanoma brought a tumor remission rate of up to 62% with a 33% complete remission (hereinafter referred to as Paper 2).
In a comprehensive analysis, the combination of oncolytic viruses and immunodetection inhibitors can significantly improve the therapeutic effect of melanoma. Here, we make a simple comparative analysis of these two articles.
- Background introduction
Talimogene laherparepvec, also called T-Vec, is a genetically modified type 1 herpes simplex virus (HSV-1). It’s the first FDA-approved oncolytic virus drug that replicates and expresses in tumor cells the granulocyte-macrophage-colony-stimulating factor(CM-CSF), an immune-activated protein, which accelerates the anti-tumor immune response and is mainly used for the treatment of advanced melanoma tumors.
Ipilimumab is a novel, fully human monoclonal antibody against CTLA-4 (cytotoxic T-lymphocyte-associated antigen 4). Blocking the immune effect of CTLA-4 can break the peripheral immune tolerance of the immune system to the self-tissue and induce or enhance the anti-tumor immune response. Ipilimumab was the first drug discovered to extend the overall survival of patients with advanced melanoma. T-Vec and Ipilimumab can enhance the activation of T cells through different mechanisms. T-Vec can increase antigen presentation and T cell activation, thereby increasing tumor-specific immune activation. Blocking of CTLA-4 by Ipilimumab can promote T cell proliferation.
Pem-brolizumab, the first PD-1 immunodetection inhibitor approved by the FDA, is expected to substantially improve the survival of patients, which has been shown highly effective in the treatment of terminal cancers t including lung cancer, kidney cancer, melanoma, head and neck cancer, bladder cancer, breast cancer, liver cancer, gastric cancer, esophageal cancer, glioma, colon cancer, and Hodgkin’s lymphoma.
- Research purposes
In paper 1, the authors evaluated differences between the efficacy of Tailmogene laherparepvec combined with Ipilimumab and that of Ipilimumab alone in a Phase II clinical study.
In paper 2, the investigators predicted that infiltration of tumors by CD8+ T cells by other immunotherapeutics might improve the efficacy of PD-1 antibodies. A combination drug program was designed to inject the oncolytic virus T-VEC directly into the melanoma lesions, and then enter the PD-1 anti-tumor drug (pem-brolizumab) intravenously to evaluate the therapeutic effect.
- Research methods
In Paper 1, patients with unresectable stages IIIB to IV melanoma were selected with no more than one prior therapy if BRAF wild-type and no more than two prior therapies if BRAF-mutant, and without autoimmune symptoms or obvious clinical immunosuppression.
Patients were randomly allocated to the combination therapy group (T-VEC + ipilimumab) and the Ipilimumab group on a 1:1 basis. In the combination group, T-VEC was started in the first week (<=4ml x 10^6 PFU/ml for the first dose and <=4mlx10^8PFU/ml every two weeks after 3 weeks), and in the 6th week Ipilimumab (3 mg/kg once every 3 weeks) was administered 4 times. In the Ipilimumab group, Ipilimumab (3 mg/ml once every 3 weeks) was used in the first week of treatment and administered 4 times. The primary clinical indicator was the objective response rate (ORR) assessed according to the immune-related response criteria.
In Paper 2, at the beginning of the trial, the patient’s tumor was injected with 4 mL x 10^6 PFU/ml of T-Vec to induce seroconversion and a protective immune response, followed by another 4 mL x 10^8 PFU/ml T-Vec 3 weeks later. Starting from the 6th week, patients received the same dose of intratumoral injection of T-Vec and intravenous injection of 200 mg of pem-brolizumab every two weeks.
- Experimental results
In paper 1, 198 patients were randomly assigned to talimogene laherparepvec plus ipilimumab (n = 98), or ipilimumab alone (n = 100). Thirty-eight patients (39%) in the combination arm and 18 patients (18%) in the ipilimumab arm had an objective response (odds ratio, 2.9; 95% CI, 1.5 to 5.5; P = .002). Responses were not limited to injected lesions; visceral lesion decreases were observed in 52% of patients in the combination arm and 23% of patients in the ipilimumab arm.
In paper 2, it’s 62% for the total response rate in the combination therapy of oncolytic agent T-VEC (talimogene laherparepvec) and PD-1 antibody drugs and in 21 patients with metastatic melanoma in this clinical trial, with a 33% complete remission, which means that their tumor has not been detected. The remission rate of this combination therapy is higher than the expected remission rate (usually about 35% to 40%) compared to the use of pem-brolizumab or T-Vec alone.
- Conclusions
- Whether it is a combination of T-VEC (talimogene laherparepve) and Ipilimumab or T-VEC (talimogene laherparepvec) and PD-1 antibody combination, it can significantly improve the treatment of melanoma.
- It is not easy to determine which treatment is better due to many differences in the patient’s baseline and background between the two experiments. If only considering the objective response data analysis of the article, in the treatment of melanoma, the combined effect of T-VEC (talimogene laherparepvec) and PD-1 antibody is better than that of T-VEC (talimogene laherparepvec) and Ipilimumab.
References:
- Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination with Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma;
- Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltrationand Improves Anti-PD-1 Immunotherapy.