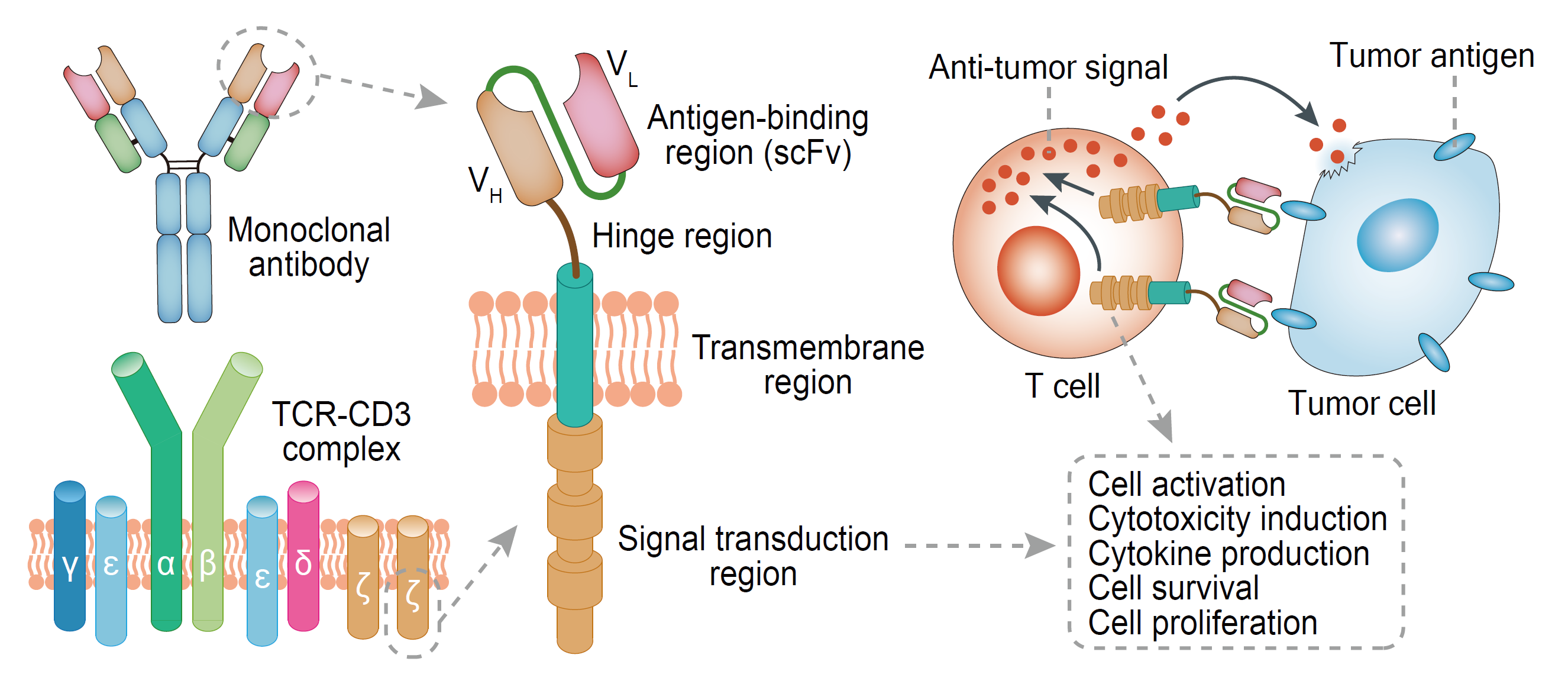
As we know, a typical design of 2rd generation Chimeric antigen receptor contains 3 functional units: a recognition domain, a transmembrane spacer domain and an intracellular signal transduction domain. Antigen recognition domain is usually an antibody scFv segment, its function is specifically recognizing the tumor associated antigen and targeting CAR-T cell to tumor cells. The intracellular signaling domain usually originates from the signal transduction subunit of T cells or NK cells such as 4-1BB and CD28, whose function is to transduce extracellular binding signal into CAR T cells and to initiate the activation of downstream signaling cascades. It is obvious that these two region is essential for CAR-T cell functional. But the function of transmembrane domain is usually neglected. Is transmembrane spacer domain necessary? Why is it important? What function it play as? Now we will take a deep look from the following two papers.
1.The Non-signaling Extracellular Spacer Domain of Chimeric Antigen Receptors Is Decisive for In Vivo Antitumor Activity
CARs consist of an extracellular antigen-binding domain that is most commonly a single chain variable fragment (scFv) of a mAb linked to intracellular signaling components such as CD3z alone or combined with one or more costimulatory domains. Research in CAR design has focused on identifying scFvs that when expressed in T cells confer recognition of malignant cells without serious toxicity to normal tissues, and on defining optimal intracellular signaling modules to activate T-cell effector functions.
Target recognition by a CAR is MHC independent and differs from that of a T-cell receptor. In MHC-restricted T-cell recognition, the fixed dimensions of the T-cell receptor and MHC molecules determine the spatial interactions of T cell and target cell, whereas with CAR-modified T cells (CAR-T cells), the interaction is influenced by the structure and density of the target molecule on the tumor and the location of the epitope that is recognized. It was appreciated that for optimal CAR-T-cell recognition, the sequences between the scFv and the T-cell membrane should provide flexibility, and the length of this spacer region may need to vary depending on the target molecule.
Some of the results from formal clinical trials demonstrate that different composition and length of the spacer domain between the scFv and the T cell membrane may result in different anti-tumor effect. But the direct comparison is difficult due to the small number and heterogeneity of patients. This study designed CD19- and ROR1-specific CARs with a modified IgG4 hinge and various components of the Fc region in the extracellular domain to examine the effect of spacer length and composition on in vitro and in vivo function.
This studies demonstrate that CD19-CARs with a long spacer from IgG4 hinge-CH2-CH3 are functional in vitro but lack antitumor activity in vivo due to interaction between the Fc domain within the spacer and the Fc receptor–bearing myeloid cells, leading to activation-induced T-cell death. In vivo persistence and antitumor effects of CAR-T cells with a long spacer can be restored by modifying distinct regions in the CH2 domain that are essential for Fc receptor binding, and modifications that abrogate binding to Fc receptors are crucial for CARs in which a long spacer is obligatory for tumor recognition as shown here for a ROR1-specific CAR. These results demonstrate that the length and composition of the extracellular spacer domain that lacks intrinsic signaling function can be decisive in the design of CARs for optimal in vivo activity.
2.Incorporation of a hinge domain improves the expansion of chimeric antigen receptor T cells
In the last 5 years, chimeric antigen receptor (CAR) T cells have emerged from bench to bedside and made headlines in clinical trials at a number of academic institutions. Multiple iterations of CARs have been developed, mainly focusing on intracellular signaling modules, which are deemed crucial for CAR design. However, the effect of non-signaling extracellular modules, such as hinge and TM domains, on the proliferation of the transduced T cells and therapeutic efficacy of CARs remains largely unclear. A hinge domain is a structure between the targeting moiety and the T cell plasma membrane; these sequences are generally derived from IgG subclasses (such as IgG1 and IgG4), IgD and CD8 domains, of which IgG1 has been most extensively used. Currently, studies of the hinge domain mainly focus on the following four aspects:
1) reducing binding affinity to the Fcγ receptor, thereby eliminating off-target activation;
2) enhancing the single-chain variable fragment (scFv) flexibility, thereby relieving the spatial constraints between tumor antigens and CARs, in turn promoting synapse formation between the CAR T cells and target cells;
3) reducing the distance between an scFv and the target epitope;
4) Facilitating the detection of CAR expression using anti-Fc reagents
To better understand the effect of the hinge domain on CAR T cells, this research generated two versions of CARs, with or without a hinge domain, targeting CD19, mesothelin, PSCA (prostate stem cell antigen), MUC1, and HER2 (human epidermal growth factor receptor 2), respectively. In vitro migration assay showed that the hinges enhanced CAR T cells migratory capacity. The T cells expressing anti-CD19 CARs with or without a hinge had similar antitumor capacities in vivo, whereas the T cells expressing anti-mesothelin CARs containing a hinge domain showed enhanced antitumor activities. Hence, this results demonstrate that a hinge contributes to CAR T cell expansion and is capable of increasing the antitumor efficacy of some specific CAR T cells. Our results suggest potential novel strategies in CAR vector design.
The spacer domain between antigen recognition domain and the signaling domain maybe small but absolutely important. It is a key component to better understand how chimeric antigen receptor works. We hope these original research about the spacer domain or so called hinge domain could be of help to our client who is interested in or working on the investigation of CAR-T cell therapy.