Nucleic Acid Aptamer
Nucleic acid aptamers are single-stranded DNA or RNA (ssDNA or ssRNA) that are able to bind specifically to target molecules by folding to form a unique tertiary structure.
The atomic structure resolution of the nucleic acid aptamer-target complex proves that the two are bound by non-covalent interaction forces such as van der Waals forces, hydrogen bonds, and base stacking forces.
Nucleic acid aptamers offer the following advantages:
- Recognition of small molecules, peptides, proteins, and cells with a wide range of targets.
- A mature and rapid screening process (ideally within one week), rapidly obtaining aptamers that bind specifically to the target substance.
- Current automated DNA/RNA synthesis technologies enable simple, cost-effective, large-scale production and automatic chemical modification of aptamers with a low batch variation.
- Nucleic acid aptamers are stable and can fully recover conformation even after thermal or chemical denaturation.
These advantages make aptamers attractive for targeted tumor therapy applications, and they have also been widely used in many other biomedical applications, including in vitro bioanalysis and in vitro diagnostics (IVD), bioimaging, novel tumor markers, and aptamer-assisted drugs.
SELEX Technology
The SELEX technology is an exponential enrichment technique for ligand phylogeny. Using this technique, nucleic acid aptamers with high affinity for the target material can be obtained from a library of random single-stranded nucleic acid sequences.
The initial library of this technique is a single-stranded DNA or RNA library consisting of 1014–1016 random oligonucleotides of 20–100 nucleotide sequences with a random region in the middle of a fixed primer sequence at both ends.
After incubation of the initial library with the target material, the bound oligonucleotides are separated from the unbound sequences and amplified by PCR, resulting in an enriched pool for the next round of screening (see Fig. 1 for the process), with targets ranging from small molecules to cellular-level biomolecules.
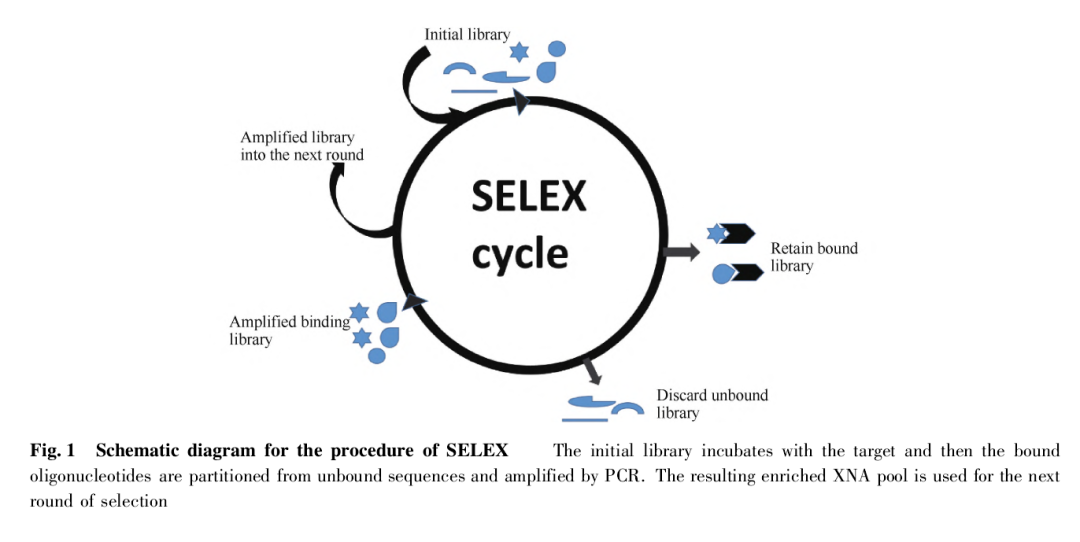
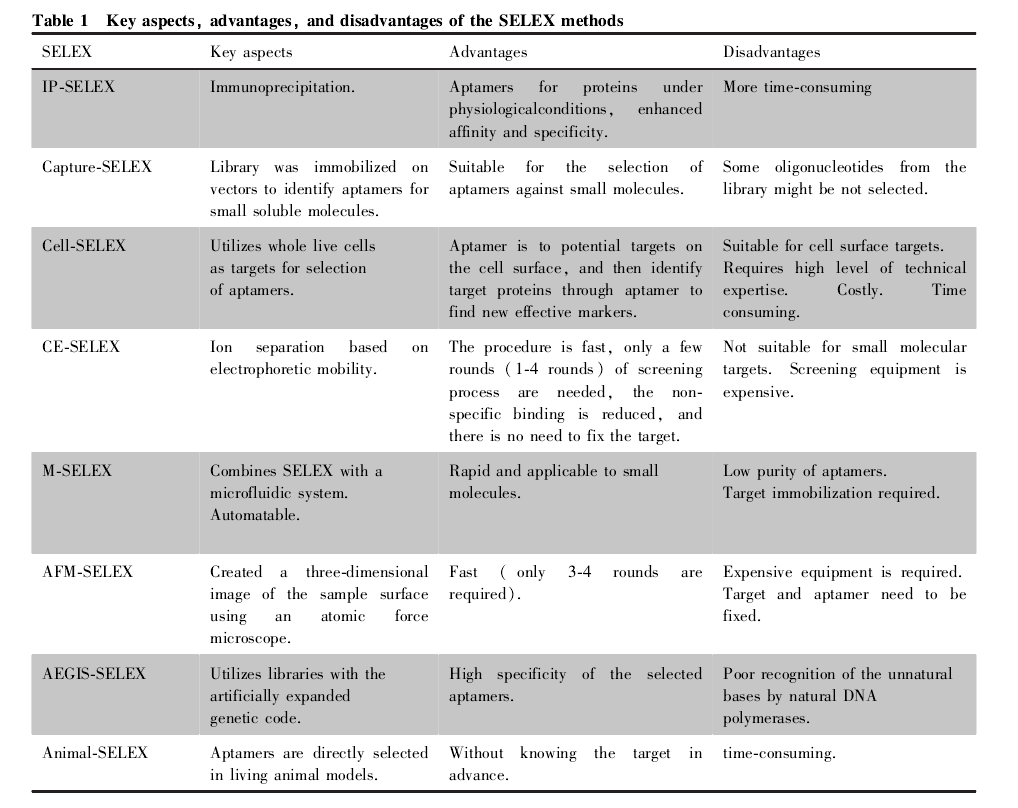
In recent years, many new methods have emerged to obtain nucleic acid aptamers more efficiently and reliably, and the key features, advantages, and disadvantages of each SELEX technique are shown in Table 1.
Nucleic Acid Aptamers and Immunotherapy
Tumor immunotherapy is based on the recognition of tumor cells by identifying cellular proteins that are expressed differently from normal cells. In a normal organism, the immune system removes tumor cells in a timely manner to maintain the organism’s homeostasis.
These abnormal proteins are often referred to as tumor-associated antigens. However, the strength of this immune response is limited, and over time, tumor cells develop mechanisms of immune recognition evasion.
These mechanisms include the expression of inhibitory ligands, which induce the downregulation of immune function through negative co-stimulation. Therefore, activation of the immune system and re-release of the suppression of the immune system, thus enhancing the function of the immune system, is the focus of immunotherapy.
- Nucleic acid aptamers as immunotherapeutic agents
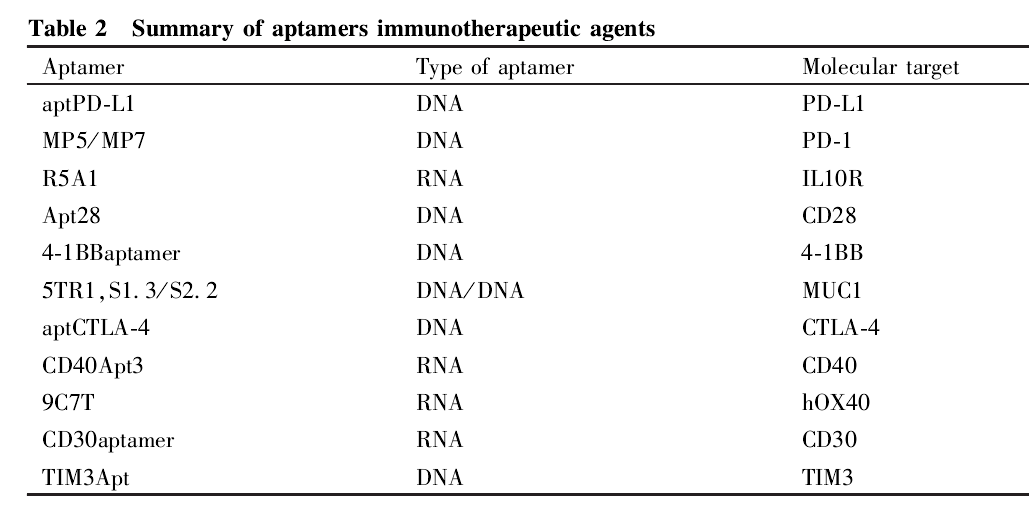
Immune checkpoint blockade therapy has opened a new era in cancer treatment. The combination of nucleic acid aptamers and tumor immunotherapy provides a new way of thinking about tumor treatment. Some studies have directly used nucleic acid aptamers as immunotherapeutic agents and achieved good results in the experiments (see Table 2).
(1) PD-1/PD-L1
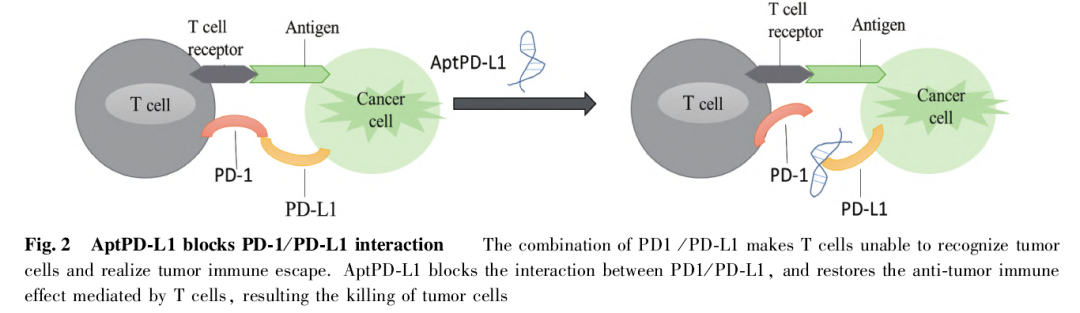
The nucleic acid aptamer PD-1 is a T cell receptor signaling co-suppressor expressed on activated T cells, and its ligand PD-L1 is expressed in both cancer cells and tumor-infiltrating lymphocytes. PD-1/PD-L1 binding inhibits T cell function, and this molecular mechanism is thought to be one of the major pathways involved in tumor immune escape.
Lai et al. obtained aptPD-L1, a 45-base DNA nucleic acid aptamer with high affinity (KD=4.7 nmol/L), which was able to block PD-L1 and inhibit PD1/PD-L1 binding through eight rounds of SELEX screening and high-throughput sequencing.
The results of mouse models showed that aptPD-L1 promoted lymphocyte proliferation in vitro and inhibited tumor growth in vivo without significant hepatotoxicity or nephrotoxicity.
aptPD-L1 was found to have significantly higher levels of infiltrating CD4+ and CD8+ T cells, IL-2, TNF-γ, interferon (IFN-γ), and C-X-C motif chemokines CXCL9 and CXCL10. Compared with the control group, CD8+ T cells in the experimental group had higher CXCR3 expression levels. In addition, the length and density of CD31+ microvessels in the tumors were significantly reduced in the aptPD-L1 treatment group.
All experimental data suggest that aptPD-L1 contributes to the restoration of T-cell function and improvement of the tumor microenvironment. These chemokines are able to act synergistically, thus attracting more T cells into the tumor tissue and creating an anti-tumor growth microenvironment. The result also suggests that aptPD-L1 can be useful as a complement therapy by taking advantage of nucleic acid aptamers (see Fig. 2).
PD-1 antibodies are now approved for the treatment of clinical melanoma and are in clinical trials for a variety of other tumor types. PD-1 monoclonal antibodies are used both as monotherapy and as part of a combination therapy regimen.
PEG-MP7 has been found to function in vivo not only as a specific PD-1 antagonist but also to promote a strong anti-tumor immune response. In addition, PD-1 DNA nucleic acid aptamer treatment is not cytotoxic and does not activate TLR9 intrinsic immune signaling.
Due to the inherent advantages of nucleic acid aptamers, including their lack of immunogenicity, low cost, long shelf life, and ease of synthesis, PD-1 nucleic acid aptamers are potential alternative therapeutic agents to antibody-based PD-1 therapies.
- Nucleic acid aptamers act as “locomotives” for targeting
Nucleic acid aptamers are well-targeted and easily modified, and when combined with drugs, they can play a full role as a “locomotive” to deliver “cargo” to its destination with precision. A review of immunotherapeutic aptamers is shown in Table 3.
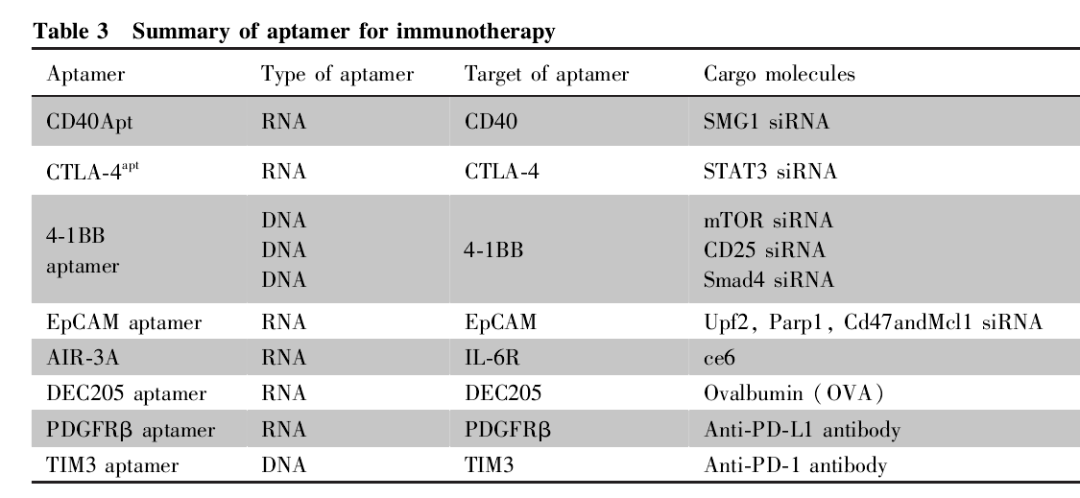
(1) Nucleic acid aptamer coupling siRNA
siRNA is a class of double-stranded RNA molecules with a length of about 20–25 base pairs, which ultimately silences the target gene to achieve therapeutic effects.
The use of nucleic acid aptamers to identify the target and then endocytosis can deliver siRNA into the cell correctly, thus exerting gene-silencing effects. Therefore, how to couple the two has become a hot research topic.
4-1BB is a costimulatory molecule expressed on CD8+ T cells after TCR stimulation. Berezhnoy et al. performed in vivo studies in mice by binding a nucleic acid aptamer targeting 4-1BB to mTORC1 siRNA in order to specifically target activated CD8+ T cells. The results showed that reduced expression of mTOR, a target of rapamycin, promoted the differentiation of effector cells into memory T cells.
The 4-1BB aptamer targets activated T cells and delivers siRNA for mTOR to the cytoplasm after internalization of 4-1BB. The intracellular siRNA inhibits the expression of mTOR, which facilitates the induced differentiation of memory T cells for their immune function. It has been shown that persistent interleukin 2 (IL-2) produced by activated CD8+ T cells in turn produces an inhibitory signal to T cells. The use of small interfering RNA (siRNA) binding to CD25 (IL-2Ra) to attenuate IL-2 in CD8+ T cells enhances T cell activation.
Rajagopalan et al. created 4-1BBaptamer-CD25siRNA couples to deliver siRNA to 4-1BB+CD8+ T cells via nucleic acid aptamer targeting. Systemic administration resulted in the downregulation of CD25mRNA only in 4-1BB+CD8+ expressing T cells and promoted their differentiation to memory T cells.
4-1BBaptamer-CD25siRNA conjugates enhance the intracellular antitumor response and the antitumor response to local radiation therapy. With this method, 4-1BBaptamer-Axin-1 siRNA couples can enhance CD8+ T cell memory differentiation and antitumor activity. These findings suggest that targeted nucleic acid aptamer siRNA conjugates can be used to modulate CD8+ T cell function and enable their development into persistent memory CD8+ T cells, thereby enhancing tumor immunity.
The tumor-associated antigen epithelial cell adhesion molecule (EpCAM), which is commonly found in epithelial cancers and is highly expressed in tissues, is selectively knocked out in EpCAM+ tumors by linking nucleic acid aptamers to siRNA chimeras (AsiCs), allowing the immune system to more easily recognize cancer cells and remove them.
(2) Nucleic acid aptamer-coupled monoclonal antibodies
Triple-negative breast cancer (TNBC) is a unique and aggressive cancer that is resistant to chemotherapy and has a high recurrence rate.
In a human TNBC mouse model, PDGFRβ promotes tumor cell growth and the metastasis of lung cancer cells. Therefore, the combination of PDGFRβ nucleic acid aptamers and PD-L1 monoclonal antibodies provides a new idea for the treatment of TNBC.
The nucleic acid aptamer enhances the antibody’s ability to inhibit tumor cell growth and lung cancer cell metastasis while also acting on tumor cells to inhibit the Akt and ERK1/2 signaling pathways, increase tumor in situ CD8+ T cells, and decrease FOXP3+ Treg cells, thus fulfilling its immunotherapeutic function.
The combination of PDGFRβ nucleic acid aptamers with PD-L1 monoclonal antibodies is a feasible strategy, providing evidence for PDGFRβ/PD-L1 combination targeting for TNBC.
In both in vitro and in vivo trials, Gefen et al. discovered that the combination of a nucleic acid aptamer targeting TIM3 with a PD-1 monoclonal antibody was effective in extending the life span of mice and that the combination regimen was more effective than treatment with RMT3-23 monoclonal antibody (targeting TIM3) alone.
Issues and Outlook
Many nucleic acid aptamers have been developed to target proteins involved in immune processes for autoimmune disease-related protein detection and imaging, and for tumor immunotherapy by enhancing the tumor immune response or deregulating immune response suppression signals.
Aptamers bound to immunotherapy can act as agonists or antagonists and thus have greater potential to replace antibodies in diagnostic and therapeutic applications of tumors. Nucleic acid aptamers have good properties, but there are still problems such as poor stability in clinical experiments, which is an important reason why nucleic acid aptamers are not widely used in the clinic.
How to enhance the conformational stability in vivo without losing the aptamer binding affinity will be the main challenge for the clinical application of nucleic acid aptamers.
The main methods to improve the stability of the aptamer include:
- Chemical modifications stabilizing the ribulose phosphate backbone at the 2-position are effective in protecting the aptamer from nuclease degradation.
- Binding to high molecular weight substances prevents rapid renal clearance, e.g., polyethylene glycol (PEG)-coupled aptamers maintain their activity by reducing elimination by the body while increasing residence time in the serum by reducing glomerular filtration.
- Locked nucleic acid (LNA) is a synthetic nucleic acid analogue containing a bridging bicyclic glycosyl group that is highly heat stable and resistant to nuclease degradation. The coupling of nucleic acid aptamers with locked nucleic acid increases the stability of the aptamer and nuclease resistance.
- Dimeric, trimeric, and tetrameric structures, all with a high molecular weight, are aptamers that have the advantage of producing high affinity and low toxicity in vitro and in vivo. The selection and development of aptamers with excellent chemical and biological properties will facilitate their application in tumor immunotherapy.
The application of nucleic acid aptamers in tumor immunotherapy is promising. A full understanding of the interaction between nucleic acid aptamers and the immune system, as well as increasing the stability of nucleic acid aptamers in vivo, will be critical to realizing their molecular immunotherapy prospects.
Article from: Chinese Journal of Biochemistry and Molecular Biology
Author: Dongmei Han, Hao Jin
Disclaimers: Creative Biolabs is intended to encourage communication and learning among colleagues in the pharmaceutical industry. This article is not intended for commercial purposes. We respect original works. The selected articles have been clearly attributed to the source and author, and the copyright is owned by the original author. If there is any infringement or other issue, please contact us for removal.
