On November 3, 2022, Iveric Bio announced that it had submitted the first part of the “rolling review” for the new drug application (NDA) of avacincaptad pegol (ACP, also known as Zimura) to the FDA. The company is expected to complete the final part of the new drug application by the end of this year. This is the second GA therapy to be marketed around the world, as well as the fourth complement C5-targeted therapy.
About Zimura
Zimura is a pegylated RNA aptamer designed to inhibit the complement C5 protein, originally developed by Archemix. In August 2007, Iveric entered into a license agreement with Archemix, obtaining the global development and commercialization rights of Zimura as an ophthalmic drug. The researchers believe that the overactivation of the complement system and C5 protein plays a key role in the development of scarring and vision loss associated with GA secondary to AMD. By blocking the activity of the C5 protein, Zimura may reduce the activity of the complement system that causes retinal cell degeneration and may slow the progression of GA. The product was granted Fast Track designation by the FDA in April 2020.
Avacincaptad pegol is a novel C5 complement inhibitor indicated for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD) and Stargardt’s macular dystrophy (SMD). As previously announced, the company received an ACP Fast Track designation from the FDA. The company immediately shared the data it had collected from the GATHER2 trial with the FDA.
Glenn P. Sblendorio, Chief Executive Officer of Iveric Bio, said: “This breakthrough designation reflects that both GATHER1 and GATHER2 met their primary endpoints and their safety profiles met the stringent criteria required. We are now focused on our full NDA submission and preparations to go public, and we look forward to working with the FDA to shorten the review period for avacincaptad pegol so that patients with AMD who are suffering from GA can benefit from a new treatment.”
About Geographic Atrophy (GA)
Age-related macular degeneration (AMD) is a common form of macular degeneration and the leading cause of blindness in the elderly. It can cause permanent vision loss if not treated promptly. AMD causes significant vision impairment or blindness in around 14 million people worldwide each year. It is divided into two stages: the early stage and the late stage. Patients in the early stage often have no clinical symptoms, while moderate to severe visual impairment occurs in the late stage.
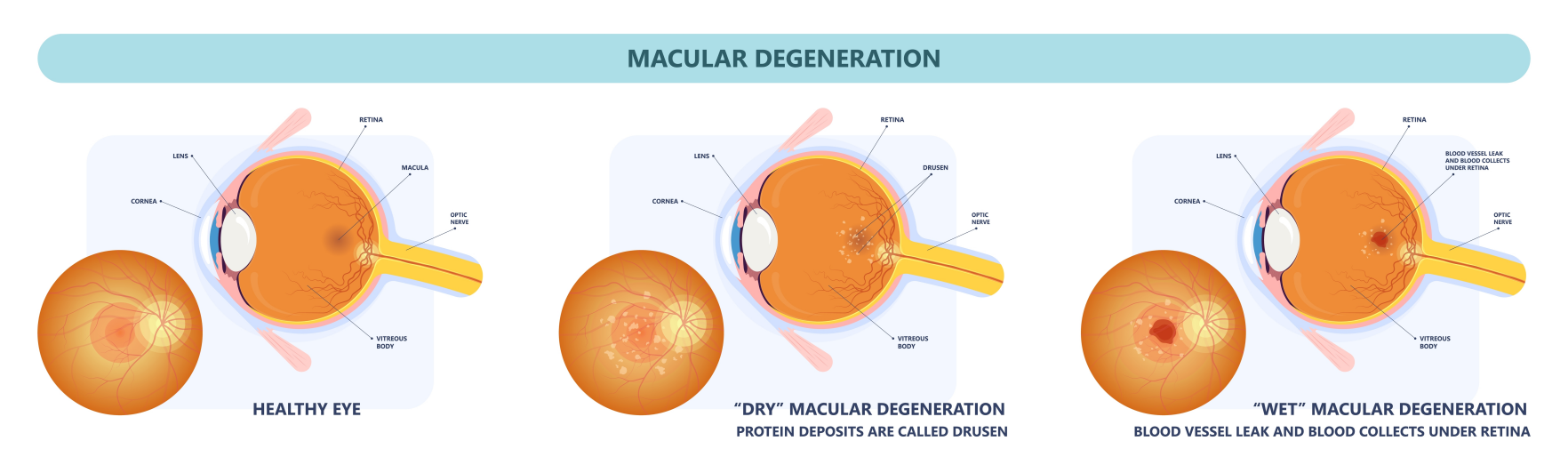
Clinically, advanced AMD is defined as having two main features: “choroidal neovascularization (CNV),” or the development of new choroidal vessels that grow without or through the retinal pigment epithelium (RPE) into the neural retina; and “progressive atrophy of the RPE,” also called “geographic atrophy (GA).” GA is also referred to as “dry” AMD and CNV as “wet” or “exudative” AMD. GA and CNV are not mutually exclusive and can appear together in the same eye. Little is known about the combined presence of GA and CNV in AMD patients.
GA is an important cause of bilateral irreversible severe functional vision loss. Many people with GA develop scotomas in the visual area even though their central vision remains normal. Thus, GA has a major impact on functional vision, quality of life, and independence in affected individuals. From the time of diagnosis, the median time to develop central GA is two and a half years, and it is expected to develop in the other eye within approximately seven years. According to a comprehensive epidemiological study published in the “Archives of Ophthalmology” in 2004, approximately 1.5 million people in the United States currently have GA.
Additionally, according to a 2015 study published in the American Journal of Ophthalmology, approximately 159,000 people in the United States suffer from GA each year. Although anti-VEGF therapies are available for the treatment of wet AMD, no FDA or European Medicines Agency (EMA) approved treatments are currently available for GA. Therefore, the treatment options for GA and many other stages of AMD, including intermediate AMD, are lacking, which is an area of urgent unmet medical need and also a major public health concern for the expanding geriatric population.
Epilogue
Eye diseases can be caused by a variety of factors, and in severe cases, eye diseases can lead to complete vision loss. The most common eye diseases that can cause complete loss of vision are those that affect the retina and optic nerve, including AMD, diabetic retinopathy, and glaucoma. In addition, many other eye diseases are less common but still represent an unmet medical need, notably orphan IRD associated with mutations in a single gene (called monogenic), which often leads to retinal degeneration and vision loss in younger patients. The diseases listed above are not only potential market opportunities but also increasingly difficult disease problems that ophthalmic drug developers must explore and investigate.
Disclaimer: Creative Biolabs focuses on promoting biological and biomedical research globally. This article is for information exchange purposes only. This article is also not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
