The complement system is an important component of innate immunity, playing a key role in defending against invading pathogens. It consists of approximately 60 soluble and membrane-bound proteins, including core components, receptors, and regulatory factors. Recent comprehensive assessments of complement function have revealed its multifaceted involvement beyond typical immune processes.
Indeed, the complement system contributes to fundamental physiologic processes. However, age-related changes, defects in complement proteins, as well as genetic variants, can disrupt complement function, leading to harmful consequences. Additionally, emerging research highlights the significant involvement of the complement system in the progression of metabolic dysfunction-associated steatosis liver disease (MASLD). Despite this pathophysiological link, the interaction between complement and MASLD remains poorly understood.
Few human MASLD cases have been analyzed, leaving questions unanswered regarding the abundance of complement components in health and disease, and the extent to which they are activated or inhibited in MASLD patients. The debate continues over whether complement activation is enhanced or suppressed in MASLD and whether the level of complement components correlates with the severity of the disease.
About MASLD
MASLD, formerly known as nonalcoholic fatty liver disease (NAFLD), has been redefined to include hepatic steatosis accompanied by cardiometabolic risk factors. This array of histopathologic findings includes what was previously known as nonalcoholic steatohepatitis (NASH), now referred to as metabolic dysfunction-associated steatotic liver disease.
MASH, the inflammatory subtype of MASLD, poses a significant risk of progression to cirrhosis and hepatocellular carcinoma (HCC). individuals diagnosed with MASH face a higher risk of mortality. The prevalence of MASLD has surged alongside the global obesity epidemic, with approximately 25-30% of the adult population worldwide currently affected. Projections suggest that by 2040, more than half of all adults may be affected by MASLD.
The progression of MASLD is influenced by a variety of factors, including diet, lifestyle choices, and the composition of the gut microbiome, all of which contribute to aberrant epigenetic modifications that drive disease progression. DNA methylation is a relatively stable epigenetic mechanism that regulates gene expression. Typically, hypermethylation silences gene expression, while hypomethylation activates it.
Therefore, studying the DNA methylome is crucial for identifying biomarkers associated with disease progression and for screening potential therapeutic targets. Research conducted on human liver specimens has revealed an association between MASLD and abnormal DNA methylation patterns.
DNA Methylome Analysis Reveals Epigenetic Alteration of Complement Genes in MASLD
Researchers from the Korea Institute of Life Sciences and Technology recently published a study demonstrating that epigenetic alterations in complement genes are correlated with the severity of MASLD. This research provides valuable insights into the mechanisms driving the progression of MASLD and suggests that inhibiting the function of certain complement proteins may offer a promising strategy for treating this condition.
Blocking the complement system shows potential as a strategy to halt the progression of MASLD. However, the exact interaction between complement dysregulation and MASLD remains to be fully elucidated. This integrative approach aims to explore the potential association between complement dysregulation and the histologic severity of MASLD.
The investigators obtained liver biopsy specimens from a cohort of 106 Koreans, including 31 controls, 17 patients with isolated steatosis, and 58 patients with MASH. An in-depth analysis of methylation changes in 61 complement genes was performed using the Infinium Methylation EPIC array. Quantitative RT-PCR and pyrophosphate sequencing were used to detect the expression and methylation status of 9 complement genes in a mouse MASH model.
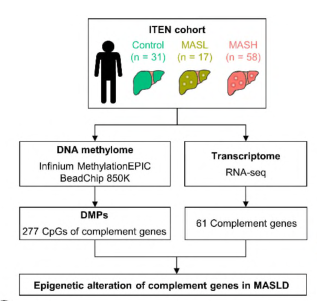
Fig. 1 Schematic diagram of the experimental design for expression and methylation analysis of complement genes.1
Methylome and transcriptome analyses of liver biopsies showed that hypermethylation and down-regulation of complement C1R, C1S, C3, C6, C4BPA, and SERPING1, as well as hypomethylation and up-regulation of complement C5AR1, C7, and CD59, were correlated with the histological severity of MASLD. In addition, DNA methylation and the relative expression of these nine complement genes in the MASH diet mouse model were consistent with the findings in human data. This study demonstrates a correlation between epigenetic alterations in complement genes and the severity of MASLD, providing valuable insights into the mechanisms driving the progression of the disease. It also suggests that inhibiting certain complement proteins could be a promising strategy for the treatment of MASLD.
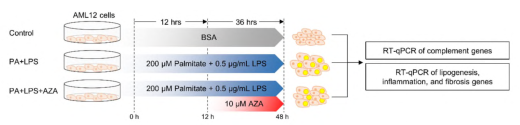
Fig. 2 Expression patterns of 9 complement genes in cells.1
In summary, this study is the first to reveal the epigenetic and transcriptional changes in hepatic complement genes during the progression of MASLD. These findings have significant implications for understanding the core mechanisms of MASLD progression and for developing targeted therapies.
With state-of-the-art technology and extensive experience, Creative Biolabs is the trusted choice for complement genetic tests. We provide the highest standards in genetic testing, utilizing the latest technology, strict quality control, and unparalleled service and reliability.
Reference:
1. Magdy, Amal, et al. “DNA methylome analysis reveals epigenetic alteration of complement genes in advanced metabolic dysfunction-associated steatotic liver disease.” Clinical and Molecular Hepatology (2024).
