The complement system is a primary barrier of the innate immune defense system formed by a series of proteins working in a highly coordinated manner to defend against microbial invasion of host tissues. In the late 19th century, Jules Bordet discovered the complement system in the blood, for which he was awarded the Nobel Prize in Physiology and Medicine in 1919. Over the next hundred years, more complement components were discovered, with the complement protein C3 being recognized as the core protein where the three activation pathways (the classical pathway, the lectin pathway, and the alternative pathway) converge.
C3 protein has long been thought to be produced primarily by hepatocytes, with the complement system functioning mainly in the blood and interstitial fluids. But does the complement system exist in the human gut, which is densely populated with microorganisms, including bacteria, archaea, fungi, protozoa, and viruses? If it exists, how does the complement system establish a harmonious coexistence with the commensal microbiota, and how does it deal with pathogens?
Dennis Kasper’s research team at Harvard Medical School published a paper in Cell entitled “Gut complement induced by the microbiota combats pathogens and spares commensals. “The paper reveals the existence of a separate complement system in the intestine that is distinct from the blood complement system. By selectively expressing specific complement components, the intestinal complement system can work in harmony with intestinal commensal bacteria while acting as a defense against pathogens.
Study of the Complement System in the Gut
The team explored the intestinal environment to determine whether a complement system exists within it and how it operates in a microbial symbiotic environment.
- Using a sterile mouse model, the team demonstrated that intestinal C3 levels are regulated by the microbiota through gut microbial transplantation experiments.
- Using single-cell RNA sequencing, the researchers found that intestinal stromal cells are the main C3-expressing cells in the gut, predominantly located in colonic lymphoid follicles.
- By establishing an in vitro culture system, the researchers demonstrated that primary intestinal stromal cells can sense microbial signals and secrete C3.
- The researchers verified this finding in humans by testing and analyzing human intestinal samples, discovering that intestinal C3 tests in humans vary depending on individual intestinal flora.
- Further studies found that colonization of the gut with Prevotella spp. increased C3 levels, suggesting that gut C3 levels can be modulated by regulating the composition of the gut microflora.
Their subsequent study revealed the mechanism of action of the intestinal complement system. During the infection stage of intestinal pathogens, the intestinal complement system activates C3 mainly through alternative pathways to generate C3b, which binds to pathogens. At the same time, C3b binds to complement receptors on neutrophils, leading to the phagocytosis of pathogens by neutrophils.
It has been shown that in the intestinal complement system, the expression levels of C5-C9, which form the membrane attack complex, are extremely low and almost undetectable even during infection. In contrast, the expression of C3 and Cfb, the main proteins of the alternative pathway, was significantly up-regulated during infection. Thus, the intestinal complement system functions mainly through phagocytosis. However, under normal physiological conditions, the number of neutrophils in the gut is relatively limited, so the complement system does not massively phagocytose the commensal microbiota in the gut.
Further studies revealed that the level of C3 in the gut was inversely correlated with the severity of host pathogen infection, offering the possibility of influencing the level of C3 by modulating the intestinal flora to prevent and treat intestinal infections.
In summary, this study is the first systematic in-depth investigation of the complement system in the intestine. It found that not only does an independent complement system exist in the intestine, but also that by selectively expressing specific complement components, the system achieves harmonious coexistence with the commensal microbial system under the regulation of the intestinal commensal microbiota. Nonetheless, the intestinal complement system is still able to act as a first line of defense for the effective clearance of intestinal pathogens, achieving precise regulation of commensal and pathogen responses.
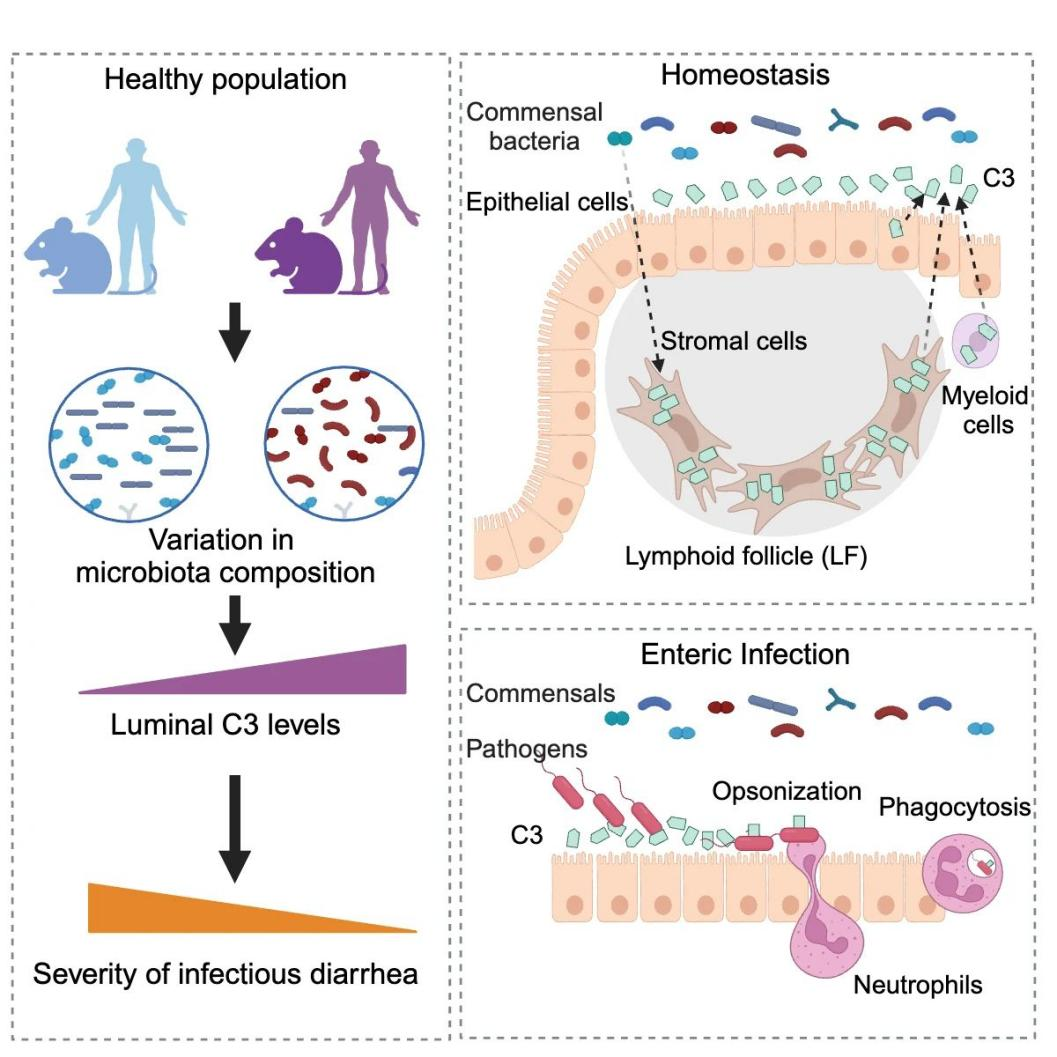
Fig. 1 Gut complement induced by the microbiota combats pathogens and spares commensals.1
This study reveals the function of the complement system on the surface of the intestinal mucosa and provides a new avenue for microbe-targeted precision medicine approaches for the prevention and treatment of intestinal diseases.
Reference:
- Wu, Meng, et al.”Gut complement induced by the microbiota combats pathogens and spares commensals.” Cell 4 (2024): 897-913.
