The complement system, as an important component of the body’s immune system, plays a key role in tumor development and therapy. Tumor immunomodulation by the complement system has a dual role in tumor development: while it may promote tumor growth, metastasis, and immune escape, it also has potential anti-tumor effects. The complex interactions between the complement system and the tumor microenvironment, as well as complement modulators, hold great promise for application in tumor therapy. Future studies will continue to explore the new findings regarding the complement system’s role in tumors and translate these discoveries into clinical practice for more effective strategies for tumor therapy.
Introduction to the Complement System
The complement system is a vital and intricate network of proteins within the body’s immune system, boasting numerous physiological functions and immunomodulatory effects. Comprising approximately 30 complement system proteins, it is categorized into three pathways: the complement activation pathway, regulatory pathway, and membrane attack pathway, forming a complete protective network. The complement system contributes to immune response and body regulation in the following ways:
- Immunoregulation: It promotes inflammatory responses, regulates immune cell activities, and clears bacteria, viruses, and other pathogens, thereby crucially maintaining the body’s internal and external stability and combating infections.
- Inflammatory response: Activation of complement proteins occurs upon encountering invasive pathogens, triggering an inflammatory response This cascade prompts leukocytes to phagocytose and kill the pathogen, while also initiating subsequent immune cell and mediator responses to create the immune reaction.
- Cytolysis: The complement system is also capable of directly disrupting cell membranes, leading to the lysis of pathogens or abnormal cells. This process involves the formation of membrane attack complexes that result in the destructionlysis of the target cell.
Overall, the complement system serves not only as an important component of the immune system but also as a crucial regulatory system for fighting infections, maintaining homeostasis, and participating in immune regulation. An in-depth understanding of the complement system’s structure, function, and regulatory mechanisms is imperative for devising therapeutic strategies for related diseases and the diagnosis and treatment of immune diseases.
The Interaction of Complement with Tumors
In the usual sense, the complement system plays a pivotal role in tumor eradication.
- Cytolytic effect: The membrane attack complex (MAC) or cytolytic complex formed after complement activation can directly bind to tumor cell surfaces, forming pores to disrupt cell membrane integrity, ultimately leading to tumor cell lysis and death.
- Promote immune cell-mediated anti-tumor effects: An activated complement system can cause the activation and phagocytosis of immune cells such as macrophages and natural killer cells, thus enhancing their ability to eliminate tumor cells.
- Induce apoptosis: Complement system activation can also induce apoptosis, or programmed cell death, through a series of signaling pathways, thereby limiting the proliferation and dissemination of tumor cells.
1. Interaction with Immune Cells
Complement proteins can activate macrophages, recruit them to tumor tissue, and induce macrophage polarization while inhibiting macrophage inflammatory vesicle activation. Additionally, complement system activation induces the accumulation and differentiation of neutrophils within tumors and promotes their neutrophil recruitment by stimulating the release of leukotriene B4 (LTB4) from epithelial and endothelial cells. Complement C5a acts as a potent chemoattractant for myeloid-derived suppressor cells (MDSCs), fostering their migration to primary tumors. Cancer cell-derived complement C5a recruits MDSCs, thereby creating a favorable immune microenvironment for lung cancer progression. Moreover, tumor-associated fibroblasts expressing C5aR can enhance cancer stem cell (CSC) enrichment and chemoresistance by secreting IL-6 and IL-8 after receiving complement signals.
Overall, the complement system may be involved in tumor growth promotion by interacting with various immune cells. A comprehensive understanding of these mechanisms will help us to better understand the complexity of tumor development and provide important clues for tumor therapeutic development targeting the complement system.
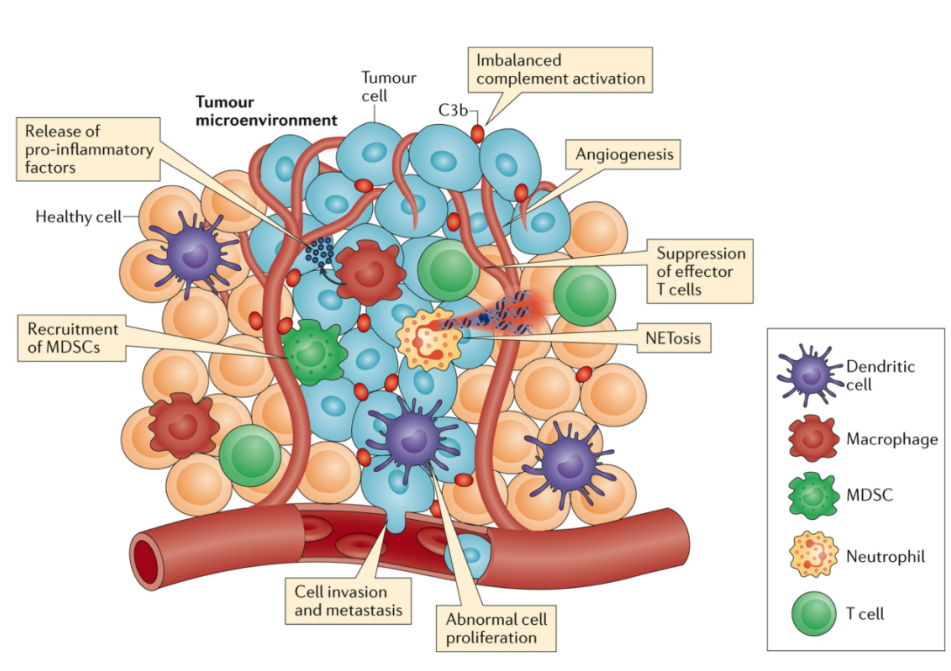
Fig. 1 Complement Activation in the Tumour Microenvironment Promotes Tumorigenesis1
2. Neoangiogenesis and Complement
The effect of complement on tumor angiogenesis is complex and the role of complement components in tumor angiogenesis needs to be further investigated. The following are some possible mechanisms:
- Promoting inflammatory response: The activated complement system can induce an inflammatory response and trigger a variety of cells to participate in the inflammatory response in the tissues surrounding the tumor. This inflammatory response promotes neoangiogenesis by releasing inflammatory mediators such as VEGF.
- Regulation of cell signaling pathways: The activated complement system regulates angiogenesis by influencing cell signaling pathways in endothelial and other cells, such as PI3K/AKT, MAPK/ERK, and other pathways, thus promoting angiogenesis.
- Enhancement of vascular permeability: Activation of the complement system can change the morphology and function of vascular endothelial cells, increase vascular permeability, provide convenient conditions for new blood vessel formation, and help tumor cells obtain more nutrients and oxygen.
3. Thrombosis, Complement, and NETs
A complex triangular relationship exists between the complement system, thrombosis, and NETosis (neutrophil extranet release), and these interactions have important implications for both tumorigenesis and progression. Activation of the complement system may both promote thrombosis and induce the NETosis process, which in turn are interconnected and jointly involved in shaping the tumor microenvironment.
First, complement system activation may trigger thrombosis by activating the coagulation cascade, which leads to platelet aggregation and fibrin deposition, forming a thrombus. These thrombi may not only provide structural support within the tumor microenvironment but also shield tumor cells, aiding in immune evasion and resistance to chemotherapy.
Second, the activation of the complement system also induces NETosis, the release of extracellular networks by neutrophils. These extracellular networks contain DNA, proteins, and cytokines that attract immune cells and mediate inflammatory responses. However, excessive or prolonged NETosis may lead to excessive inflammation and exacerbate the immunosuppressive state of the tumor microenvironment, fostering tumor progression and metastasis.
Taken together, the interactions between the complement system, thrombosis, and NETosis form a dynamic network with profound effects on the tumor microenvironment.
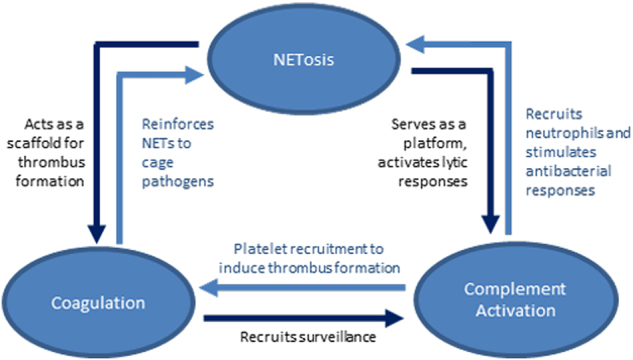
Fig. 2 Summary of the Interplay Between NETosis, Complement Activation, and Coagulation2
Complement in Tumor Therapy
1.C1q Complex Inhibitors
One notable inhibitor is the protease inhibitor C1 inhibitor (C1-INH), FDA-approved for treating hereditary angioedema. C1-INH effectively inhibits the activation of the classical pathway while preserving the functional activation of other initiating pathways. Studies suggest its potential role in the treatment of neuroblastoma.
2. C5aR1 Antagonists
The C5aR1 antagonist PMX205, a cyclized hexapeptide, antagonizes the C5a complement receptor. PMX20 treatment significantly reduces the percentage of MDSCs in the colon and blood, while increasing CD8+ T cell percentage in the blood, mucosa-associated lymphoid tissues, and tumors. This results in a significant blockade of colorectal cancer (CRC) growth. Other C5aR antagonists, such as PMX53, effectively reduce tumor size and enhance the efficacy of anticancer chemotherapy in mouse models.
3. Anti-C5aR1 Antibody
Avdoralimab (IPH 5401) is a fully human IgGκ monoclonal antibody that targets complement C5aR1, preventing its interaction with C5a. Used in complement-driven inflammatory diseases and solid tumor studies, avdoralimab is currently investigated in clinical trials in combination with the anti-PD-L1 antibody Durvalumab for the treatment of advanced solid tumors (non-small cell lung cancer and hepatocellular carcinoma).
Despite advancements, challenges, and limitations remain in understanding the complex network established between complement and tumors. For example, despite our accumulating knowledge about the role of complement in cancer, the activation molecules that trigger the complement cascade in cancer cells are largely unknown. Due to the high heterogeneity of human cancers, different activation pathways and mechanisms may be involved, necessitating tailored strategies for different tumor types. The role of complement at specific stages of tumor progression must also be considered. In summary, complement-related tumor therapeutic strategies remain a promising yet challenging field, offering great hope for a successful fight against cancer.
References:
- Reis, Edimara, et al. “Complement in cancer: untangling an intricate relationship.” Nature Reviews Immunology18.1 (2018): 5-18.
- de Bont, Cynthia M., Wilbert C. Boelens, and Ger JM Pruijn. “NETosis, complement, and coagulation: a triangular relationship.” Cellular & molecular immunology1 (2019): 19-27.
