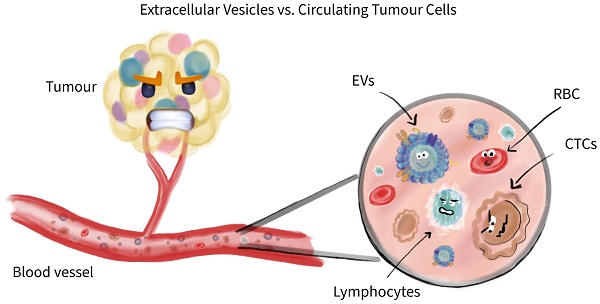
Traditional liquid biopsies for cancer primarily rely on the detection of some recognized markers. However, during clinical use, many markers have limitations in statistical significance and robustness , leading to the removal of an increasing number of liquid biopsy biomarkers from cancer diagnosis or other related clinical guidelines. Researchers from the Copernicus University in Torun, Poland, published an article discussing that extracellular vesicles (EVs) and circulating tumor cells (CTCs) can be called true liquid biopsies. They compared the characterization, quantification, antigen information, downstream applications, and other aspects of EVs and CTCs, believing that they have complementary value in cancer diagnosis. This review, titled “Extracellular Vesicles and Circulating Tumor Cells – complementary liquid biopsies or standalone concepts?”, was published in the journal Theranostics on August 1.
As early as 1966, Wichelhausen et al. published the earliest report on liquid biopsy, which obtained the patient’s cell tissue and was then further cultured and analyzed accordingly. In 1990, another important study showed that prostate-specific antigen (PSA) in serum samples correlated with prostate cancer tumor volume and differentiation and benign prostatic hyperplasia volume. A highly regarded and influential 1991 study in the New England Journal of Medicine established the gold standard for prostate cancer screening using PSA fluid. In 1994, the U.S. Food and Drug Administration approved PSA combined with a digital rectal examination (DRE) to detect prostate cancer. Another liquid biopsy cancer biomarker once thought to have the potential to take hepatocellular carcinoma (HCC) screening and diagnosis to another level is alpha-fetoprotein (AFP). This biomarker has even been recommended for inclusion in multiple international and national guidelines. Unfortunately, AFP has proven to be insensitive, as it is elevated in only 40-60% of HCC cases, especially early in the disease.
In addition to these two protein-based liquid biopsy cancer biomarkers, more liquid biopsy biomarkers for cancer have been developed subsequently. Especially in the past 15 years, “tumor circulation,” including circulating tumor cells (CTC), extracellular vesicles (EV), cell-free tumor DNA (ctDNA), cell-free DNA (cfDNA), circulating tumor RNA (ctRNA), and tumor-induced platelets (TEP), has come into focus. All these biomarkers fall within the category of liquid biopsy or are considered part of precision medicine. In this review, researchers specifically discuss the differences and similarities between EVs and CTCs used as liquid biopsy tools, comprehensively outlining their limitations and advantages in cancer screening and diagnosis.
Researchers believe that in the case of cancer liquid biopsy, CTCs and EVs may be the most promising candidates for achieving powerful, high-sensitivity, and specific applications in the future. CTCs have been approved by the Food and Drug Administration for the diagnosis of certain types of solid cancers because CTCs are mainly derived from epithelial solid tumors with metastasis, while EVs are highly experimental markers that have not been fully developed and therefore hold great promise.
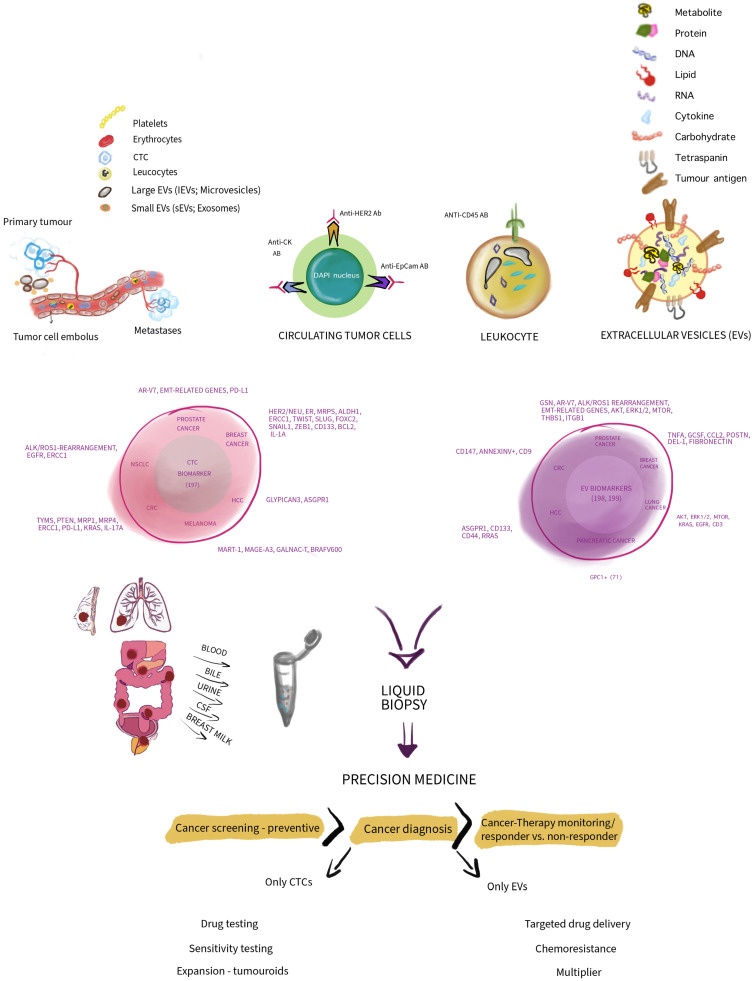
Researchers conducted an in-depth discussion and comparison of CTCs and EVs. Interestingly, both CTCs and EVs can be viewed as tools for tumor cells to fight against normal tissues in our body, but they are also tracers for cancer screening, diagnosis, and treatment monitoring. As shown in the figure below, the figure shows the independent capabilities of CTC and EV, which are not shared between the two. CTCs may be used in various ways as tumor-like components for testing drug sensitivity. EVs may be used for vectors for cancer treatment, which may be a promising vision that requires extensive research. EVs may also be used as cancer screening. Since during cancer evolution, cancer cells use EVs and CTCs to promote cancer survival and gain an advantage, we should also take advantage of CTCs and EVs and not just regard EVs and CTCs as simple biomarkers. Both EVs and CTCs orchestrate important oncogenic processes, but it appears that EVs are more involved in initiating carcinogenesis, whereas CTCs are generated later. However, both have the same goal: cancer evolution. So, which one is better for a liquid biopsy, EV, or CTC? This question is not important. For highly personalized medicine, or as the first step in early cancer screening, the real winner will be if it can help every cancer patient eliminate tumors and secondary diseases.
This review mainly describes the concepts of CTCs and EVs in an artistic comparative manner, highlighting and summarizing their differences in composition. It proposes several biomarkers for correlating CTCs and EVs with various cancers, demonstrating how they are applied as biomarkers in precision oncology and related downstream applications. For more details, readers are encouraged to refer to the original paper for reading.
Reference:
Słomka A, Wang B, Mocan T, et al. Extracellular Vesicles and Circulating Tumour Cells – complementary liquid biopsies or standalone concepts?. Theranostics. 2022;12(13):5836-5855. Published 2022 Aug 1. doi:10.7150/thno.73400
Related Services:
Exosomal cfDNA Isolation and Profiling Service