Although extracellular vesicles (EVs) play a role in intercellular communication, their various populations and secretion mechanisms have not been fully characterized. It remains unclear how and to what extent EVs form as intraluminal vesicles within endosomal compartments (exosomes) or as vesicles (ectosomes) at the plasma membrane (PM). Recently, a research group led by Clotilde Théry published an article in Nature Communications, tracking the intracellular transport of EV markers CD9 and CD63 from the endoplasmic reticulum to their respective residency compartments—the PM and late endosomes.
Transient colocalization at two sites was observed before their final separation. CD9 and a PM-stabilized mutant of CD63 were released more abundantly in EVs than CD63 itself. Consequently, in HeLa cells, ectosomes are secreted in greater quantities than exosomes. By comparing proteomic analyses and responses to endosomal pH neutralization, several surface proteins potentially specific to exosomes (such as LAMP1) or ectosomes (such as BSG and SLC3A2) were identified. This study paves the way for molecular and functional differentiation between exosomes and small ectosomes across all cell types.

All cells release membrane-enclosed vesicles into their environment, collectively known as EVs. These EVs contain a specific set of lipids, nucleic acids, and proteins originating from their parent cells, allowing them to transfer a range of complex information to nearby or distant cells. EVs can form through direct outward budding from the PM of prokaryotic and eukaryotic cells. In eukaryotic cells, EVs can also originate as intraluminal vesicles within multivesicular bodies (MVBs) of the endocytic pathway, which are subsequently secreted when these compartments fuse with the PM. To differentiate terminology, the term “exosome” is recommended exclusively for MVB-derived EVs, rather than for all small EVs. On the other hand, PM-derived EVs are referred to by various names, such as microvesicles, microparticles, or ectosomes—the latter term being used in this study to specifically denote PM-derived EVs, while other terms may refer to any EV type.
Due to their formation at distinct subcellular sites, exosomes and ectosomes may carry different specific sets of cargo and may therefore fulfill different functions. However, distinguishing between them is challenging due to the presence of similarly sized exosomes and ectosomes in biological fluids or cell-conditioned media, along with the absence of specific protein markers or fully distinct tools to separate exosomes from ectosomes.
Over the past two decades, several tetraspanins, particularly CD63, CD81, and CD9, have been used as markers for exosomes due to their accumulation in small EVs relative to whole-cell lysates and the steady-state accumulation of CD63 within MVBs. However, recent studies have also observed their presence in other types of EVs as well. By capturing EVs specifically carrying CD63, CD9, or CD81 and then analyzing their protein composition and the enrichment of endosomal markers, researchers proposed that EVs containing only CD9 or CD81, without CD63, may not form within endosomes (and thus are ectosomes), while those carrying CD63 along with one or both of the other tetraspanins likely correspond to endosome-derived exosomes. This observation was made using EVs released by primary human immune dendritic cells, which complicates direct validation of the model through cell biological analyses.
This study aims to determine the actual exosomal or ectosomal nature of EVs containing different tetraspanins. HeLa cells were used as the model system, as this cell line is well-suited for the experimental manipulations required to address cell biology questions. To identify the subcellular origin of EVs released by these cells, the researchers needed to track the intracellular trafficking of tetraspanins in a time-controlled manner, from their initial synthesis in the endoplasmic reticulum (ER) to their secretion within EVs. CD63 and CD9 were adapted to the RUSH (Retention Using Selective Hooks) system, a system previously employed to track and control the trafficking of various transmembrane and secretory proteins, thus enabling the identification of atypical secretion pathways. This approach also allowed for the synchronized release of EVs containing CD9 or CD63, facilitating the characterization of a more uniform mixture of newly synthesized EVs.
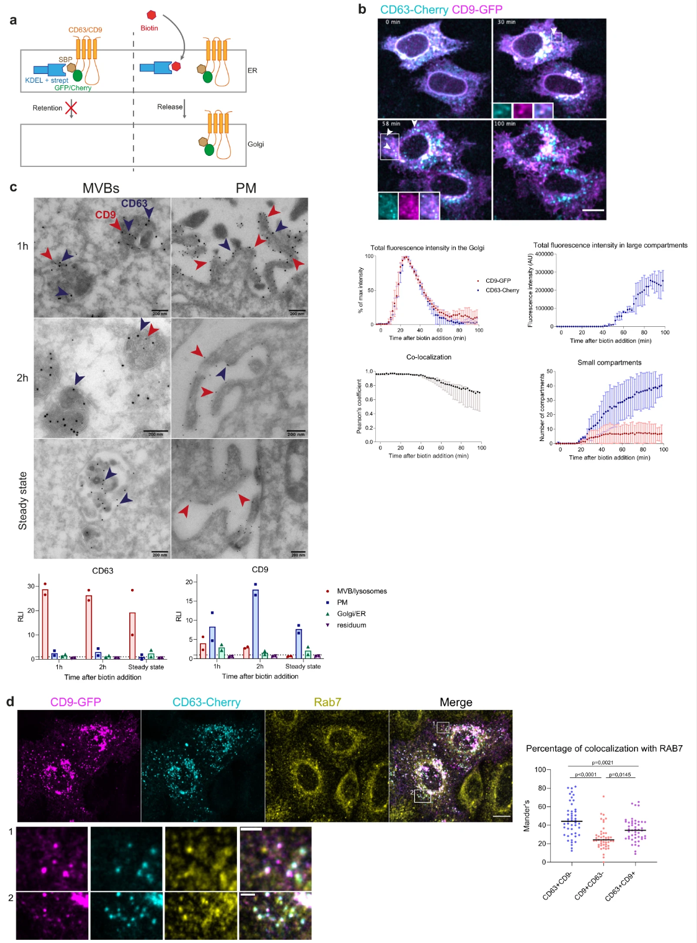 Fig. CD63 and CD9 transiently co-localize in multivesicular bodies and at the plasma membrane.
Fig. CD63 and CD9 transiently co-localize in multivesicular bodies and at the plasma membrane.
CD63 and CD9 transiently colocalize at MVBs and the PM
The findings indicate that both CD63 and CD9 can be released within small ectosomes formed at the plasma membrane. In HeLa cells, exosomes constitute a minor subset of small EVs (sEVs), carrying CD63 and other late endosomal molecules such as LAMP1/2, and their secretion is particularly sensitive to endosomal pH neutralization. BSG and SLC3A2 were identified as specific markers for ectosomes secreted from HeLa cells, which, in contrast, are not affected by endosomal pH neutralization. Interestingly, CD81, another tetraspanin commonly used as an exosome and/or small EV marker, behaves more similarly to CD9 than to CD63. The specific markers and molecular mechanisms distinguishing exosomes from ectosomes identified in this study will pave the way for further research to decode their respective functions.
Reference:
Mathieu, Mathilde et al. “Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9.” Nature communications vol. 12,1 4389. 19 Jul. 2021, doi:10.1038/s41467-021-24384-2
Related Services:
