Therapeutic approaches based on extracellular vesicles (EVs) are attracting significant attention in drug delivery, immunotherapy, and regenerative medicine. However, the clinical translation of EVs is hindered by challenges such as limited yield, functional heterogeneity, and inadequate targeting specificity. Enhancing the inherent functions of EVs and endowing them with additional functionalities holds promise for accelerating their clinical application. Bioorthogonal click chemistry has garnered widespread interest due to its ability to modify EVs in a controlled and specific manner without disrupting their intrinsic structure. This approach was further highlighted by the Nobel Prize in Chemistry in 2022, underscoring its advantages. Numerous studies have already demonstrated the substantial potential of bioorthogonal labeling in EV separation, imaging, and therapy.
Recently, scientists published a review titled “Engineering extracellular vesicles for diagnosis and therapy” in Trends in Pharmacological Sciences. The paper primarily introduces the latest advancements in engineering EVs using bioorthogonal click chemistry. It outlines labeling strategies for engineered EVs and discusses their advantages, focusing on applications in EV separation and purification, diagnostics, and therapeutic interventions. Notably, the review emphasizes the use of bioorthogonal glycan metabolic engineering in vesicles for targeted in vivo therapy. The article also provides insights into the future prospects of EV engineering technologies.
 Therapeutic approaches based on extracellular vesicles (EVs) have garnered substantial attention in the fields of drug delivery, immunotherapy, and regenerative medicine. To address the challenges limiting the clinical translation of EVs, engineering modifications are necessary. However, conventional methods such as centrifugation, ultrasound, and genetic engineering are often time-consuming and can damage the structural integrity of EVs. Bioorthogonal click chemistry offers unique advantages in EV engineering, particularly through copper-free click chemistry, which primarily includes strain-promoted azide-alkyne cycloaddition (SPAAC) and inverse electron-demand Diels-Alder (iEDDA) reactions. These methods avoid the potential toxicity associated with metal catalysts. This innovative approach provides controllability, specificity, and targeting capability, allowing for rapid reactions while minimizing interference with other biochemical processes in vivo.
Therapeutic approaches based on extracellular vesicles (EVs) have garnered substantial attention in the fields of drug delivery, immunotherapy, and regenerative medicine. To address the challenges limiting the clinical translation of EVs, engineering modifications are necessary. However, conventional methods such as centrifugation, ultrasound, and genetic engineering are often time-consuming and can damage the structural integrity of EVs. Bioorthogonal click chemistry offers unique advantages in EV engineering, particularly through copper-free click chemistry, which primarily includes strain-promoted azide-alkyne cycloaddition (SPAAC) and inverse electron-demand Diels-Alder (iEDDA) reactions. These methods avoid the potential toxicity associated with metal catalysts. This innovative approach provides controllability, specificity, and targeting capability, allowing for rapid reactions while minimizing interference with other biochemical processes in vivo.
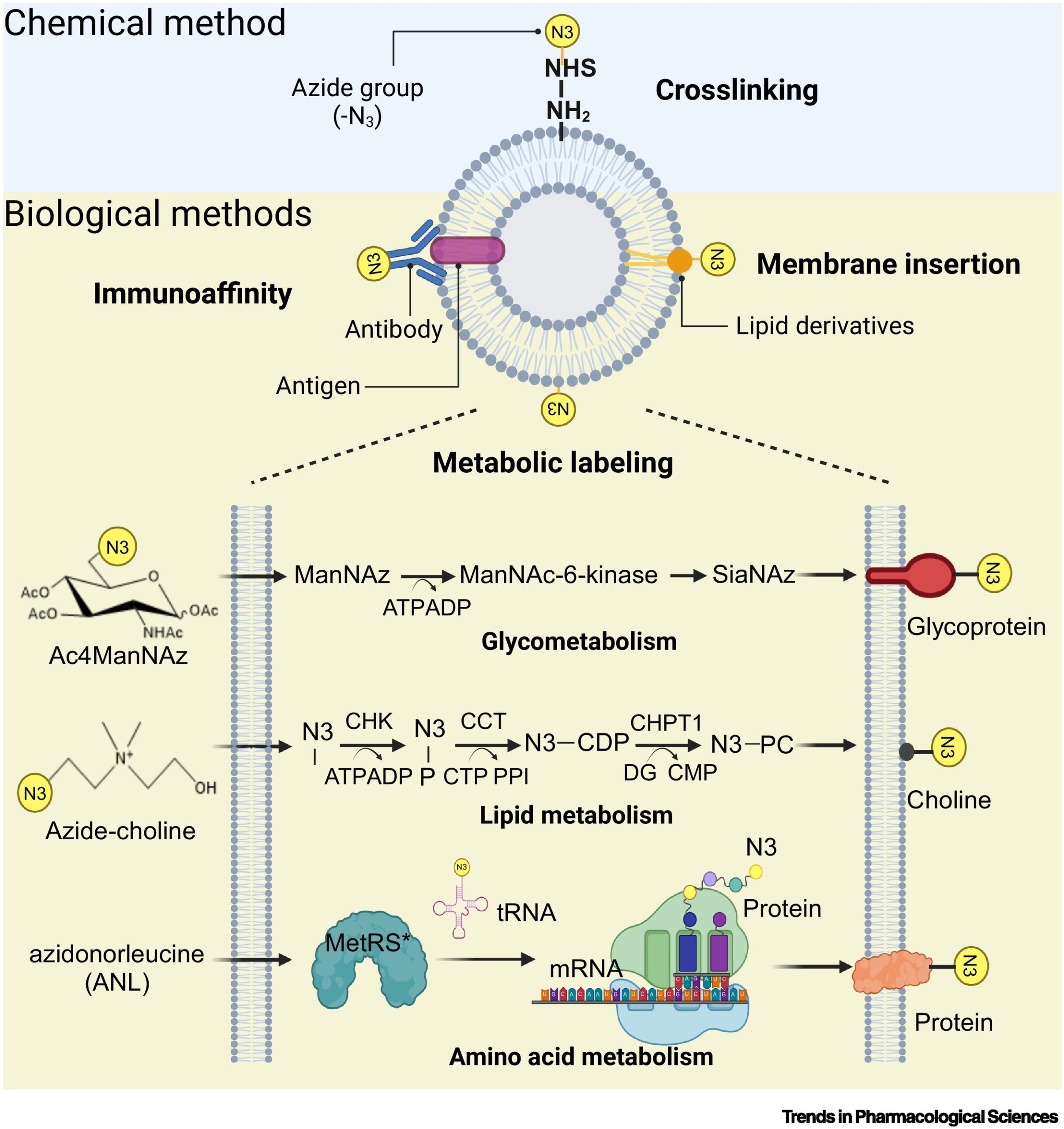
To achieve bioorthogonal reactions between EVs and biological systems, engineering modifications are primarily achieved through three biological labeling methods: chemical cross-linking and immunoaffinity, membrane insertion, and metabolic labeling. Immunoaffinity methods, which rely on EV surface proteins and antibodies/aptamers, provide high specificity but are limited by high separation costs and low yields. In contrast, membrane insertion naturally embeds lipids or lipid-mimicking molecules into the EV membrane. While membrane insertion is more broadly applicable and cost-effective, it tends to have relatively lower specificity. Metabolic engineering enables the natural modification of cell surfaces with sugars, lipids, and amino acids containing bioorthogonal functional groups (such as azides, alkynes, and aldehydes) through metabolic activities. Among these methods, metabolic engineering ensures high specificity with minimal disruption to EV integrity, facilitating in vivo applications.
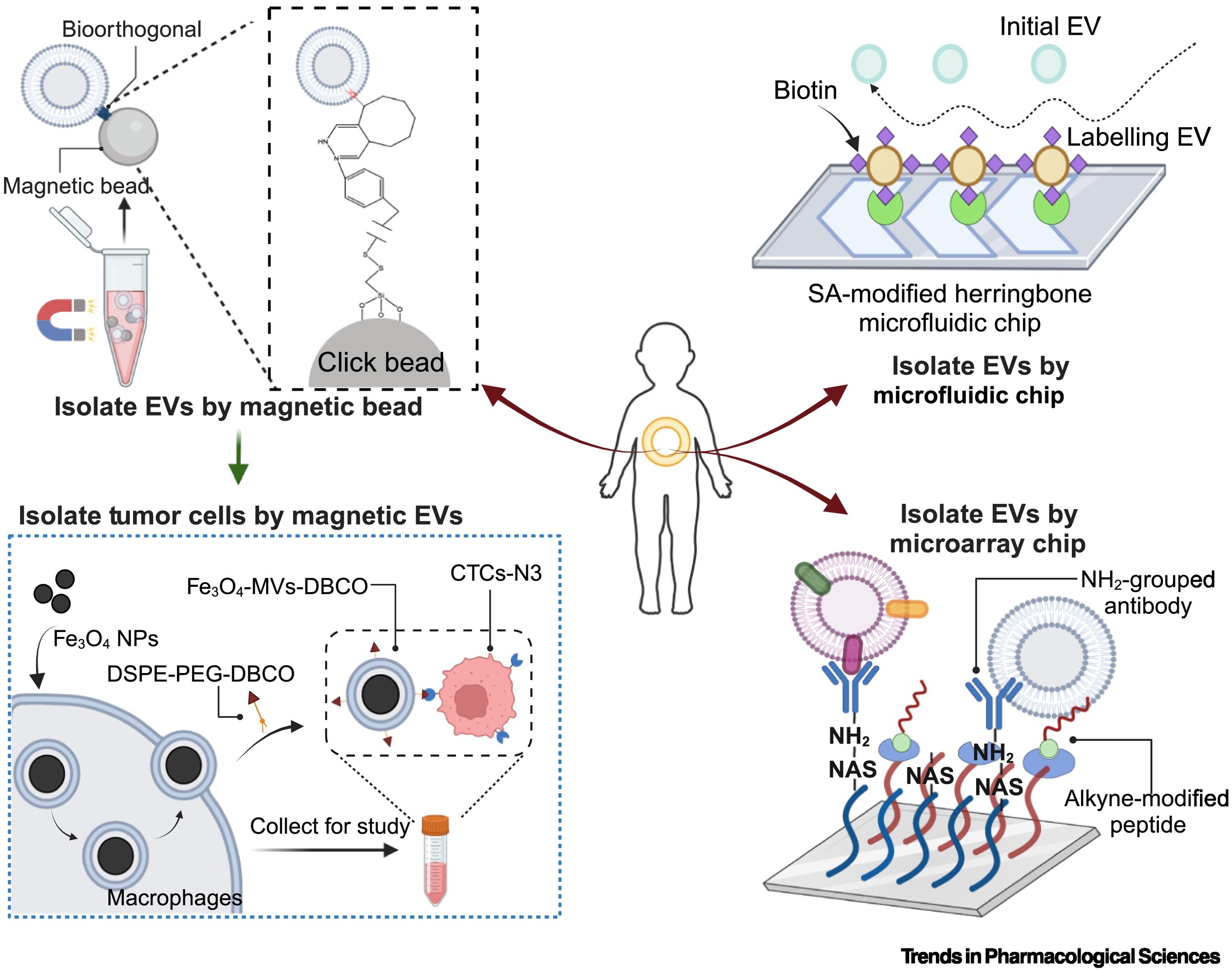
 First, considering that large-scale production of EVs is a key challenge for clinical approval, the use of magnetic beads, microfluidic chips, and microarray chips allows for orthogonal connections with engineered EVs, enabling the isolation of desired EV populations. This separation approach can reduce heterogeneity and facilitate scalable production.
First, considering that large-scale production of EVs is a key challenge for clinical approval, the use of magnetic beads, microfluidic chips, and microarray chips allows for orthogonal connections with engineered EVs, enabling the isolation of desired EV populations. This separation approach can reduce heterogeneity and facilitate scalable production.
 Secondly, engineered EV nanoprobe systems enable real-time monitoring of EV distribution, transport, and function within the body. This bioimaging technology utilizes SPAAC reactions to link EVs with fluorescent molecules, offering non-invasive and highly specific imaging that effectively overcomes current limitations in tracking and imaging EVs within cells and tissues.
Secondly, engineered EV nanoprobe systems enable real-time monitoring of EV distribution, transport, and function within the body. This bioimaging technology utilizes SPAAC reactions to link EVs with fluorescent molecules, offering non-invasive and highly specific imaging that effectively overcomes current limitations in tracking and imaging EVs within cells and tissues.
For early cancer diagnosis, click-chemistry-based electrochemical biosensors provide a reliable, convenient, and sensitive method to address the challenge of low exosome concentrations in the body fluids of early-stage disease patients. Despite some limitations, these biosensors have significant clinical potential, offering valuable information for disease diagnosis, particularly in early tumor detection and prognosis monitoring.
Finally, a major bottleneck in EV-based therapeutic approaches is limited targeting capability. Bioorthogonal chemistry not only extends the diagnostic capabilities of EVs but also enhances their targeting specificity. Through drug multifunctionalization or glycan metabolic engineering, EV drug delivery systems can be used to treat various diseases, including cancer, arthritis, ischemic stroke, and heart disease. In recent years, EV drug delivery has expanded from small molecules to nanozymes, peptides, and cells, enabling EVs to deliver drugs with active or passive targeting abilities. However, the effectiveness of ligand-targeting strategies is limited by the availability and heterogeneous distribution of receptors within tumor cells. Fortunately, bioorthogonal glycan metabolic engineering broadens orthogonal targeting by engineering functionalized glycoproteins at tumor sites, thus improving in vivo therapeutic efficacy. This method allows for precise labeling of tumors and promotes orthogonal drug-tumor binding through click reactions. This interaction inhibits the formation of tumor-derived exosomes (TDEs), prevents their uptake by liver Kupffer cells, and disrupts communication with distant tumors, effectively suppressing metastasis. This highlights the potential of click chemistry in anti-metastasis strategies and targeted EV-based therapies.
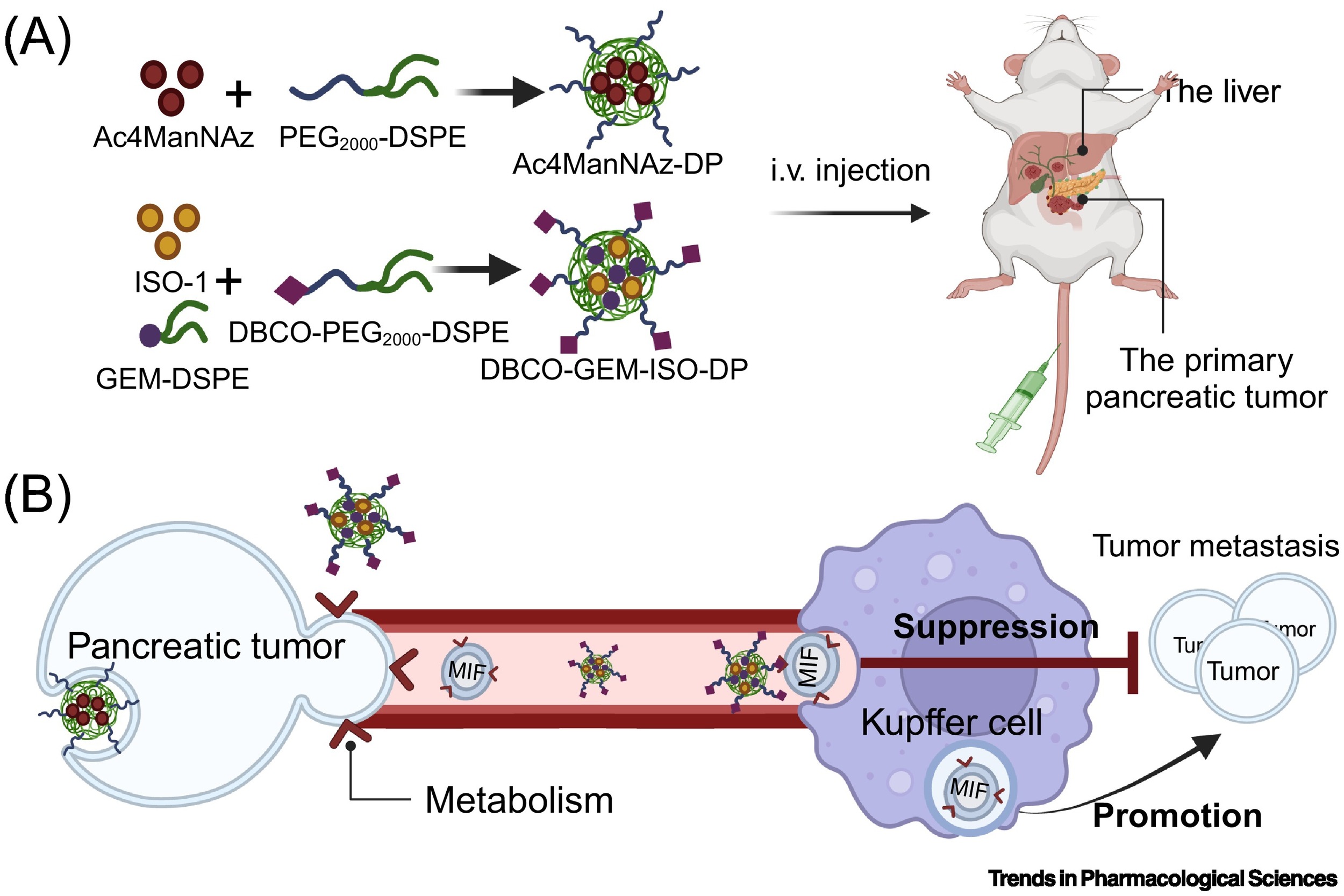 In summary, bioorthogonal labeling of vesicles holds extensive potential in applications such as EV separation, bioimaging, and targeted therapy, providing essential tools for EV-related research across fields from chemical biology to drug delivery. However, the application of bioorthogonal labeling faces several challenges and limitations, such as potential vascular damage from intratumoral Ac4ManNAz injections, azidosugar leakage, or inconsistent labeling. Enhancing labeling efficiency for tumors or EVs may be achieved by optimizing the chemical structure of natural molecule derivatives, such as adjusting the acyl side chain length in sialic acid analogs, developing Ac4ManAz that responds to tumor microenvironments, or constructing bioresponsive disulfide self-assembling entities.
In summary, bioorthogonal labeling of vesicles holds extensive potential in applications such as EV separation, bioimaging, and targeted therapy, providing essential tools for EV-related research across fields from chemical biology to drug delivery. However, the application of bioorthogonal labeling faces several challenges and limitations, such as potential vascular damage from intratumoral Ac4ManNAz injections, azidosugar leakage, or inconsistent labeling. Enhancing labeling efficiency for tumors or EVs may be achieved by optimizing the chemical structure of natural molecule derivatives, such as adjusting the acyl side chain length in sialic acid analogs, developing Ac4ManAz that responds to tumor microenvironments, or constructing bioresponsive disulfide self-assembling entities.
To advance clinical applications, an in-depth understanding of the metabolic pathways involved in bioorthogonal labeling is essential for evaluating in vivo behavior. Additionally, the commercialization of bioorthogonal-functionalized EVs requires standardized purification processes and quality control measures.
Reference:
Fei, Zhengyue et al. “Engineeringextracellular vesicles for diagnosis and therapy.” Trends in pharmacological sciences vol. 45,10 (2024): 931-940. doi:10.1016/j.tips.2024.08.007
Related Services:
