Extracellular vesicles (EVs) are a highly heterogeneous class of membrane vesicles that participate in regulating various biological processes by transporting proteins, lipids, nucleic acids, and metabolites. According to differences in size and generation pathways, EVs can be divided into exosomes, microvesicles, apoptotic bodies, and other vesicles (such as migratoria, mitochondrial vesicles, and RAB22A-induced extracellular vesicles, R-EVs, etc.). Exosomes are single-layer vesicles with a diameter of 40-160nm released by the fusion of multivesicular bodies with the plasma membrane, while microvesicles are produced by direct budding outward from the cell membrane. According to the latest guidelines from the International Extracellular Vesicle Association, exosomes and small microvesicles are collectively referred to as small extracellular vesicles (sEVs).
Recently, a research team published a review entitled “Extracellular vesicles as modifiers of epigenomic profiles” in Trends in Genetics (2024 Jun 5:S0168-9525(24)00109-4). This paper systematically summarizes the latest progress in understanding the interaction between EVs and cellular epigenetic profiles. It emphasizes the significant role of epigenetic-related factors in the biogenesis and secretion of EVs, explains the function of EVs in DNA, RNA and histone modifications, and discusses the clinical application of EVs as diagnostic markers and epigenetic drug delivery carriers.

Epigenetic Factors Involved in Regulating EV Biogenesis
In order to accurately transmit information, the biogenesis of small extracellular vesicles (sEVs), cargo sorting, and uptake by recipient cells are precisely regulated by multiple mechanisms, such as histone and DNA modifications, post-translational modifications of non-coding RNAs, and proteins. (Figure 1).
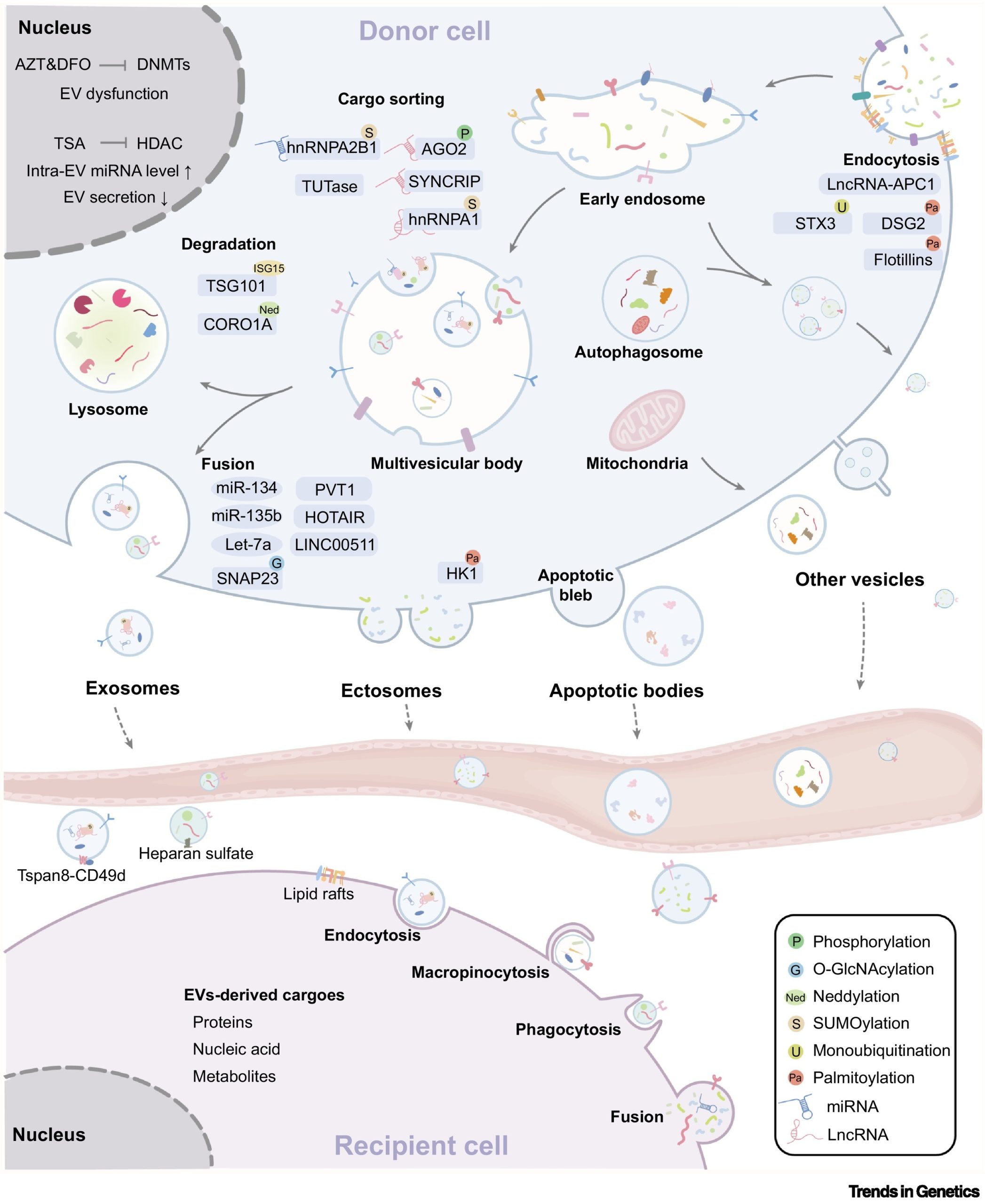 Figure 1. Epigenetic factors and protein post-translational modifications dynamically regulate EV biogenesis.
Figure 1. Epigenetic factors and protein post-translational modifications dynamically regulate EV biogenesis.
The Role of EVs in Epigenetic Remodeling
As a critical medium for intercellular communication, EVs are widely involved in various biological processes, including reshaping the epigenetic map of cells. DNA methylation is a common epigenetic modification in eukaryotes and a typical feature of heterochromatin, dynamically regulated by DNA methyltransferases (DNMTs) and Fe (II) and α-ketoglutarate-dependent dioxygenases TETs. RNA methylation, the main type of RNA chemical modification, is added by “writers,” removed by “erasers,” and recognized by “readers.” These proteins coordinate to dynamically regulate the methylation spectrum in cells, maintaining normal cell function. EVs can reshape the methylation patterns of recipient cells by directly transporting “cargo” such as mRNA of these proteins or non-coding RNA targeting these proteins, thereby regulating essential biological processes such as replication and apoptosis of recipient cells.
Histone modifications, including histone methylation and acetylation, mainly occur at the lysine residues of histone H3 or H4. These modifications are dynamically regulated by protein methyltransferases (HMT), lysine-specific demethylases (KMT), histone acetyltransferase (HAT), and histone deacetylases (HDAC). EV-delivered long noncoding RNAs (lncRNAs) such as UFC1, LNMAT2, and BCYRN1 can recruit HMT to increase histone methylation. In addition, EVs can regulate the expression of “writers” and “erasers” of histone modifications by delivering miRNA and mRNA, thereby reshaping the epigenetic landscape (Figure 2).
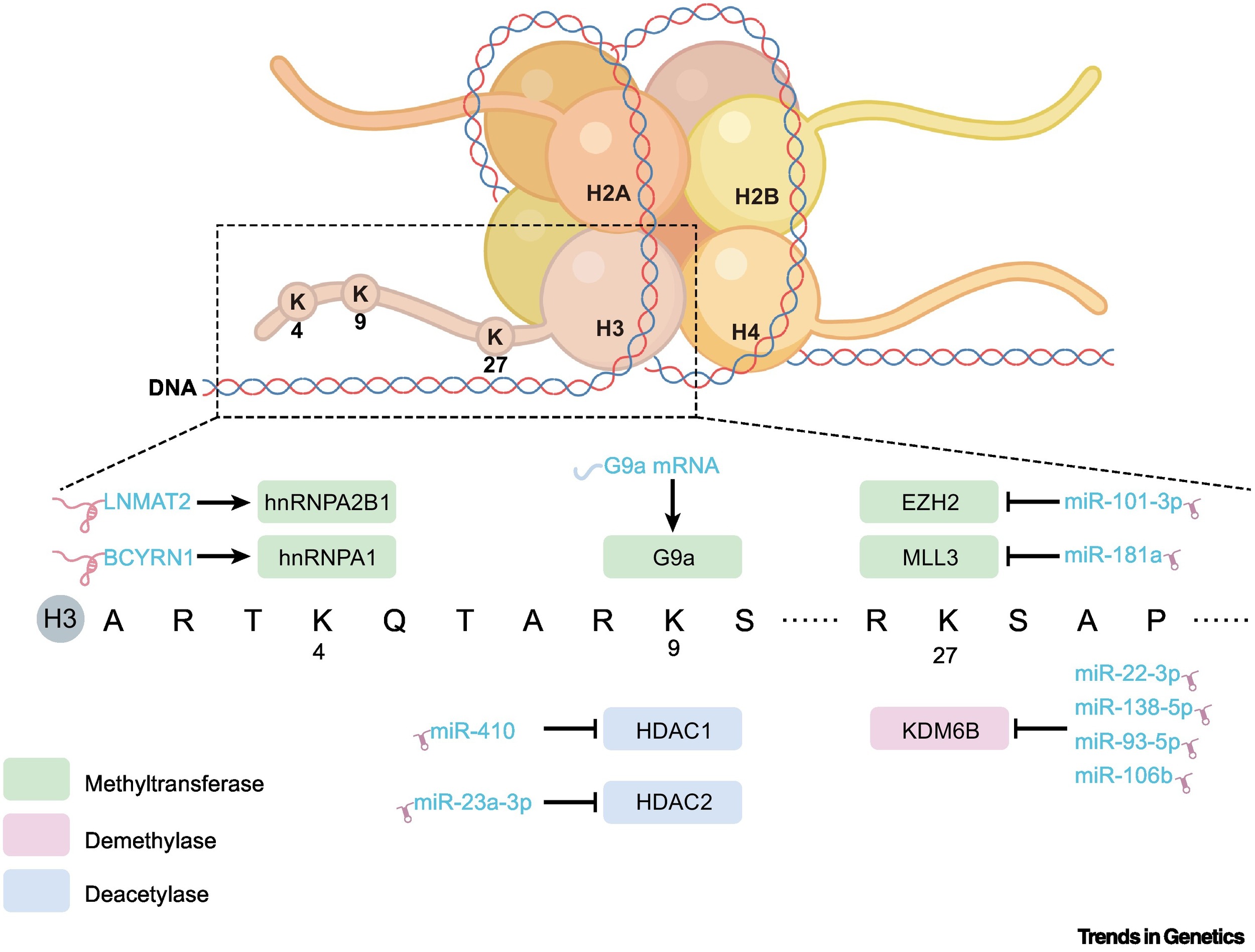 Figure 2. Biological functions of EVs in histone modification
Figure 2. Biological functions of EVs in histone modification
Application of EVs in Disease Diagnosis and Treatment
Changes in the epigenetic profile of the mother cells that secrete EVs can lead to a diversity of EV-encapsulated cargo patterns. While this brings challenges to the research and application of EVs, it also provides opportunities for the application of EVs in disease diagnosis. Analyzing “fingerprints,” such as EV epigenetic cargo extracted from patient body fluids, is a potential strategy for rapid disease diagnosis. At the same time, because EVs can cross the blood-brain barrier and have low immunogenicity, they are often considered ideal carriers for delivering epigenetic drugs. Genetic manipulation can transform donor cells to produce engineered EVs loaded with specific cargo, which can then be delivered to recipient cells to reshape their epigenetic profiles and treat diseases.
Future Outlook
EVs have gradually attracted attention as important mediators of intercellular communication, especially as emerging regulators of epigenetics. An increasing number of studies have demonstrated the mutual regulatory effects between EVs and the epigenome under both physiological and pathological conditions. In the future, comprehensive screening methods, including epigenomic analysis, are needed to explore the epigenetic factors involved in EV secretion or uptake. Delivering therapeutic cargo to reverse pathological epigenetic patterns in recipient cells may be a promising strategy. However, many challenges remain to be addressed. For example, the accuracy of engineered EV delivery and retention time in vivo need improvement. Delivering key enzymes related to epigenetic “writers,” “erasers,” and “readers” may be an ideal strategy, but potential side effects must be considered. The heterogeneity of EVs and the lack of specific markers also complicate the application of EV delivery for epigenetic drugs. As the field of EV biology rapidly develops, advanced technologies for single EV characterization, such as single EV imaging, flow cytometry, and multi-omics analysis, will help to deeply explore EV heterogeneity and significantly improve the accuracy and efficiency of EV drug delivery in future clinical applications.
Reference:
Haifeng Zhou et al. “Extracellular vesicles as modifiers of epigenomic profiles.” Trends in Genetics. Jun 5:S0168-9525(24)00109-4. doi: 10.1016/j.tig.2024.05.005
Related Services:
Therapeutic Application of Exosomes
