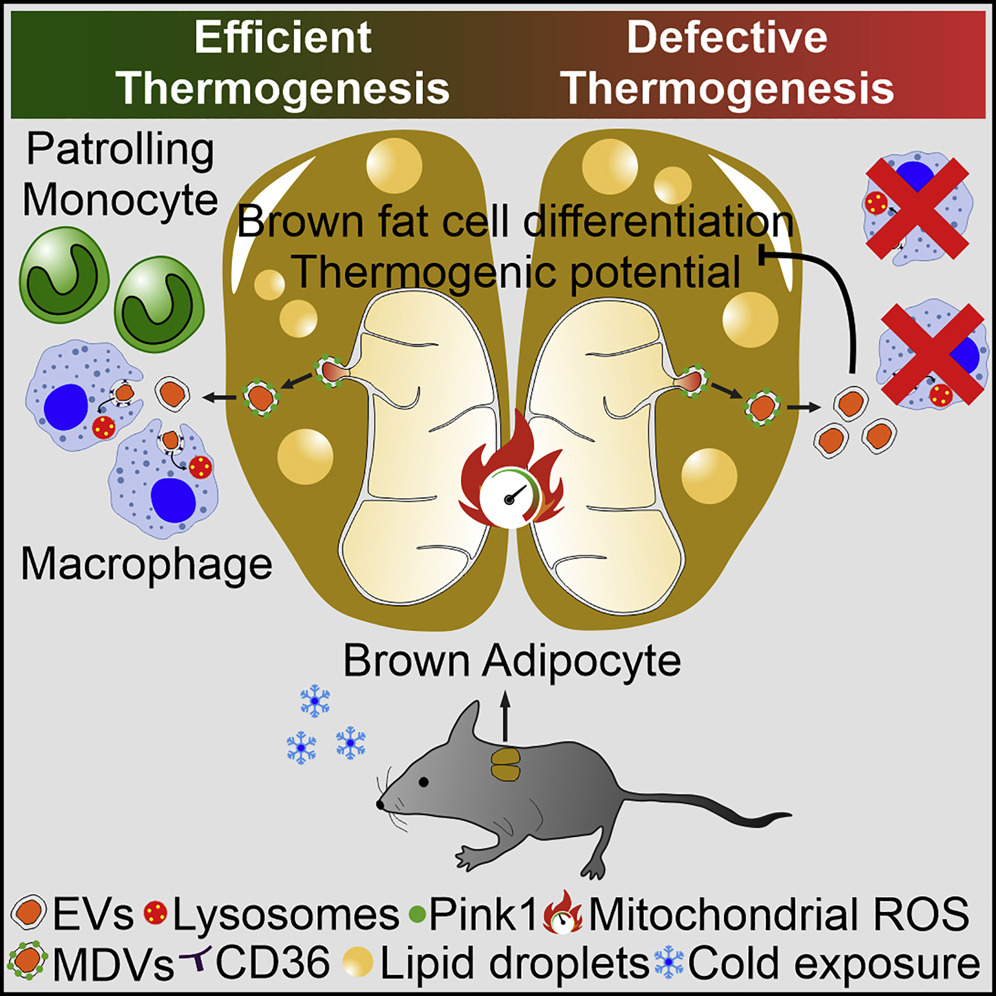
Recent findings has uncovered that mitochondria can be transferred between cells to control metabolic homeostasis. Although the mitochondria in brown adipocytes occupy a significant portion of the cell’s volume and undergo reorganization to maintain thermogenesis, it remains unclear whether intercellular mitochondrial transfer occurs in brown adipose tissue (BAT) and if it influences adaptive thermogenesis. Recently, an article published in the journal Cell Metabolism reveals that brown adipocytes under thermogenic stress release extracellular vesicles (EVs) containing oxidatively damaged mitochondrial fractions to avoid failure of the thermogenic program. When these mitochondria-derived EVs are reabsorbed by brown adipocytes, they reduce peroxisome proliferator-activated receptor-γ (PPARγ) signaling and the levels of mitochondrial proteins, including UCP1. The removal of mitochondria-derived EVs through the phagocytic activity of BAT-resident macrophages is crucial for maintaining BAT physiology. In vivo depletion of macrophages leads to the abnormal accumulation of extracellular mitochondrial vesicles in BAT, thereby impairing the thermogenic response to cold exposure. These findings highlight the essential homeostatic role of tissue-resident macrophages in maintaining mitochondrial quality control within BAT.

Brown adipose tissue (BAT) has been implicated in heat production during cold exposure. Cold exposure induces a coordinated activation of mitochondrial metabolism and dynamics, as well as oxidative stress, requiring BAT’s involvement in mitochondrial quality control processes. These processes include the selective degradation of non-functional mitochondria to maintain mitochondrial integrity. In other organs with high metabolic activity, such as the heart, mitochondrial components are extruded through the generation and release of mitochondria-derived vesicles (MDVs) to maintain mitochondrial heat production. This pathway, induced by mild oxidative stress, supports the biogenesis of MDVs that bud from mitochondria and contain specific cargo proteins. The selective packaging of mitochondrial fractions into extracellular vesicles (EVs) has been observed in multiple physiological contexts and is particularly induced during mitochondrial damage. Recent studies have demonstrated that tissue-resident macrophages actively uptake and clear extracellular mitochondria released by different cell types. However, it remains unknown whether this process occurs in BAT and if it plays a role in regulating adaptive thermogenesis.
Recently, studies have shown that adipocytes in white adipose tissue (WAT) can transfer mitochondria to macrophages, a process that may help maintain metabolic homeostasis, thereby limiting WAT expansion and obesity pathogenesis. Mice with impaired mitochondrial transfer to macrophages exhibit lower energy expenditure, while macrophage depletion impairs thermogenesis in subcutaneous adipose depots. In the heart, the exhaustion or insufficient phagocytic capacity of tissue-resident macrophages leads to defective clearance of extracellular mitochondria from tissues, resulting in tissue inflammation, accumulation of abnormal mitochondria, and metabolic alterations. Although these observations suggest that mitochondrial transfer to macrophages regulates tissue homeostasis, evidence for this immunometabolic crosstalk in BAT is lacking.
This study demonstrates that brown adipocytes release oxidatively damaged mitochondrial fractions via EVs to control mitochondrial integrity and maintain thermogenic potential. Brown adipocyte-derived EVs are taken up by BAT-resident macrophages in vivo. Macrophage depletion induces the accumulation of mitochondrial EVs, reduces the expression of mitochondrial proteins, and suppresses BAT thermogenic responses to cold exposure. These data delineate an adipocyte-to-immune cell material transfer axis that participates in mitochondrial quality control to maintain BAT function and homeostasis.
Research highlights:
- Brown adipocytes eliminate damaged mitochondrial fractions via EVs
- Thermogenic stimulation increases the release of mitochondrial EVs
- EVs exert a negative autocrine effect on brown adipocyte thermogenesis
- bMAC actively absorbs mitochondrial EVs to ensure optimal BAT thermogenesis
Limitations:
A limitation of this study is that brown adipocyte-derived EVs are heterogeneous, with some containing and others not containing mitochondrial fractions. More studies are needed to determine how EVs with or without mitochondria differentially regulate BAT function. Using bone marrow transplantation and MitoFat mice, the authors found that BAT-resident macrophages appear to be the primary recipients of adipocyte-derived mitochondria in BAT. However, these in vivo systems cannot distinguish the specific mechanisms or pathways of mitochondrial transfer. Furthermore, studies involving in vivo macrophage depletion may affect BAT metabolism and function through pathways unrelated to the clearance of damaged brown adipocyte mitochondria in EVs. Genetic tools that specifically disrupt the release or capture of EVs containing mitochondria are lacking and need to be developed to understand how this process regulates BAT function and the metabolic alterations observed in obesity, type 2 diabetes, cancer, and aging.
Reference:
Rosina M, Ceci V, Turchi R, et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022;34(4):533-548.e12. doi:10.1016/j.cmet.2022.02.016
Related Services:
Exosomal Protein Isolation and Profiling Service
