Exosomes are secretory vesicles with a diameter of about 30 to 150 nanometers that play an important role in human health and disease. To better understand how cells release these vesicles, researchers from Johns Hopkins University studied the biogenesis of the most abundant human exosome marker proteins, the exosomal tetraspanins CD81, CD9, and CD63. The results showed that endocytosis inhibits the vesicular secretion of these proteins, and CD9 and CD81 disrupt endocytosis. In addition, the exosome biogenesis factor syntenin promotes the vesicular secretion of CD63 by preventing its endocytosis. Other endocytosis inhibitors also induce the plasma membrane accumulation and vesicular secretion of CD63. Finally, CD63 is an expression-dependent inhibitor of endocytosis that triggers the vesicular secretion of lysosomal proteins and the clathrin adaptor protein AP-2 mu2. These results indicate that the vesicular secretion of exosome marker proteins occurs mainly through a pathway independent of endocytosis. The relevant content was published online on May 10 in the internationally renowned comprehensive academic journal Science Advances under the title “Endocytosis blocks the vesicular secretion of exosome marker proteins”.

Exosomes are small extracellular vesicles, approximately 30 to 150 nanometers in diameter, that share the same topology as cells and are enriched with specific exosomal marker proteins, especially exosomal tetraspanins. In mammals, exosomes are abundant in all biological fluids and can transmit signals and molecules between cells, playing a role in various physiological and disease processes. In addition, all cell types ubiquitously produce exosomes and other small extracellular vesicles, making them valuable as biosensors of tissue and organ health or disease. The biological normality of exosomes also makes them ideal nanovesicles for the delivery of vaccines, biologics, and other drugs. Therefore, understanding the biogenesis of exosome-sized secretory vesicles has become a major focus in the biomedical field.
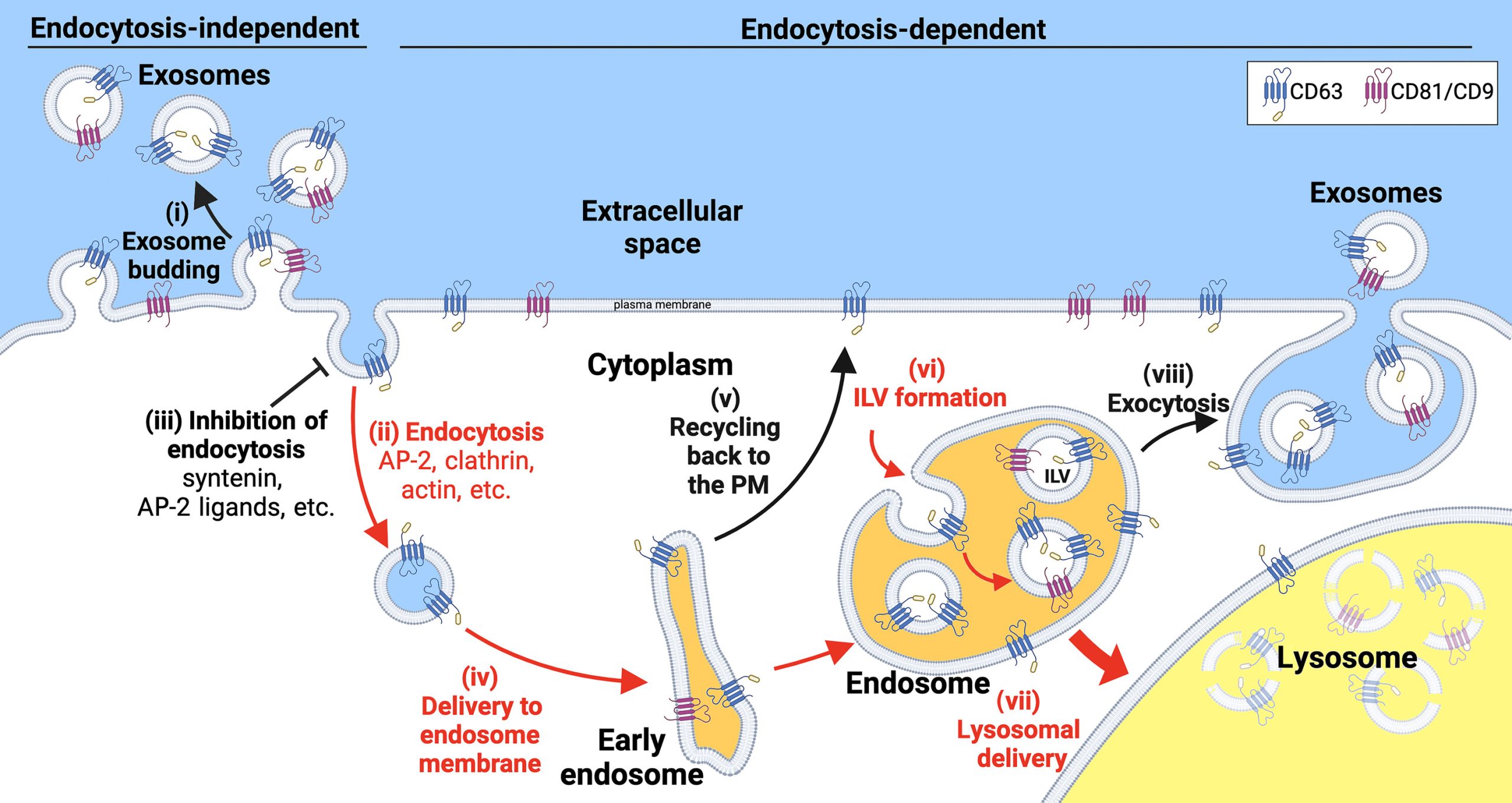
It is generally accepted that exosomal proteins bud from cells via a multistep, endocytosis-dependent pathway. This process involves the delivery of exosomal marker proteins to endosomes, loading these proteins into nascent intracellular lumenal vesicles (ILVs), and releasing the ILVs as exosomes when endosomes (also called multivesicular bodies) containing the ILVs fuse with the plasma membrane. This model has been extended to include CD63 and core proteins as recruitment factors for syntenin, syndecan-syntenin and CD63-syntenin complexes as recruitment factors for the protein Alix. Alix, in turn, recruits the ESCRT (endocytic structure-stimulating receptor) machinery to the endosomal membrane to drive ILV formation. This model is based on established protein interactions and is consistent with current understanding that ESCRTs promote outward vesicle budding (outward = away from the cytoplasm) and general membrane closure.
Given that other organelle biogenesis pathways have been elucidated by studying their most abundant proteins, researchers have investigated exosome biogenesis by studying the most abundant exosomal proteins. In a recent side-by-side comparison of 24 human exosome marker proteins, researchers found that the exosomal tetraspanins CD81, CD9, and CD63 were more abundant in exosome-sized EVs than the other 21 marker proteins tested, such as syntenin, Alix, and the ESCRT protein TSG101. This result makes sense on several levels. First, CD81, CD9, and CD63 were the first proteins shown to be enriched in exosomes. Second, they have been used as exosome marker proteins for decades. However, initial studies on the trafficking and vesicle secretion of CD81, CD9, and CD63 did not support the current mainstream model of exosome biogenesis. Specifically, researchers found that CD81 and CD9 reside on the plasma membrane and bud from cells 15- and 5-fold more efficiently, respectively, than CD63, which is constantly endocytosed from the plasma membrane and resides in endosomes. Furthermore, attaching an endocytic signal to CD9 prevented CD9 from budding from cells, whereas mutating CD63’s endocytic signal induced its budding from cells. In short, these observations suggest that there is a large gap between current models of exosome biogenesis and the actual mechanisms by which cells bud exosome marker proteins.
To further explore this gap, researchers investigated the effects of endocytic signals, endocytic inhibitors, and their expression levels on the vesicular secretion of CD81, CD9, and CD63. The results showed that endocytosis strongly inhibited the exosome secretion of all these highly abundant exosome marker proteins and triggered their destruction in the presence of CD81 and CD9. Moreover, six mechanistically different endocytic inhibitors were found to induce the vesicular secretion of CD63, which has an endocytic signal, but had no effect on the vesicular secretion of CD81, which lacks an endocytic signal. Additionally, high-level expression of CD63 was shown to directly bind the clathrin adaptor protein AP-2 subunit mu2, inhibit endocytosis, induce plasma membrane accumulation and vesicular secretion of lysosomal proteins, and trigger the vesicular secretion and cellular depletion of the AP-2 subunit mu2. These findings, along with other results, support the notion that exosome marker proteins are cleaved from cells to the plasma membrane and endosomal membranes primarily through a pathway independent of endocytosis.
Reference:
Ai Y, Guo C, Garcia-Contreras M, et al. Endocytosis blocks the vesicular secretion of exosome marker proteins. Sci Adv. 2024;10(19):eadi9156. doi:10.1126/sciadv.adi9156
Related Services:
Exosomal Protein Isolation and Profiling Service
