Extracellular vesicles (EVs), including exosomes, are cell-derived nano or micrometer-sized monolayer or multilayer membrane structures. EVs contain bioactive cargoes such as nucleic acids, lipids, proteins, and carbohydrates, and play a key role in intercellular communication. The bioactive cargoes of EVs make them promising therapeutic agents for various diseases. In addition, the surface of EVs mediates in vivo transport and site-specific delivery, allowing EVs to be used as drug delivery systems for small molecules and biopharmaceuticals.
An ideal EV-based therapeutic and/or drug delivery system should possess the following properties: i) maintain structural and biomolecular integrity before administration and when exposed to the biological environment, and ii) enable targeted delivery, including site-specific interactions at the organ, cellular, and subcellular levels. For synthetic nanoparticles, site-specific delivery generally improves with prolonged circulation time. However, prolonged circulation of cytotoxic drugs may lead to leukocyte damage, resulting in hematotoxicity. Preclinical studies have shown that intravenously injected EVs are cleared from the blood circulation within minutes. The necessity for EVs to persist in the bloodstream is unclear. It is possible that EVs, due to their more complex surfaces compared to clinically approved synthetic nanoparticles, may exhibit superior immune evasion and targeting properties. In this case, EVs may require shorter circulation times to interact efficiently with their targets.
EVs have similar bioactive properties to the cells from which they are derived, but offer several advantages over cell therapies, including improved safety due to the elimination of the risk of malignant growth and vascular occlusion. In addition, EVs are easier to handle and store than cells. Several EV clinical trials have been completed and more are underway to treat various diseases. Encouragingly, neither autologous nor allogeneic EV trials have shown toxicity. Furthermore, plasma transfusions, which are frequently performed in the clinic, result in the transfer of trillions of EVs and generally do not result in adverse events, further indicating the biocompatibility of allogeneic EVs. Animal studies have also shown that the administration of human EVs does not induce (immuno)toxicity.
Although EV therapeutics and drug delivery platforms have shown good safety profiles in preclinical and clinical studies, the unexpected immunogenicity of EVs (beyond the absence of immunotoxicity) remains largely unexplored. This Perspective focuses on adverse immune interactions. Readers are referred to other literature for studies that intentionally induced EV immunogenicity, such as EV-based vaccines and immunotherapy.
Compared with autologous EVs, xenogeneic and allogeneic EVs may show enhanced clearance by the innate and adaptive immune systems, especially after repeated administration. In the case of the adaptive immune system, EV-associated donor antigens bind to major histocompatibility complex (MHC) molecules, triggering an immune response. Notably, MHC molecules are enriched on EVs compared with the cells of origin. Independently of the adaptive immune system, the mononuclear phagocyte system can recognize xenogeneic and allogeneic EVs as foreign, for example, due to polymorphisms in self-recognition ligand-receptor pairs on macrophages. Whether this ability of the innate immune system to immediately discriminate between self and xenogeneic/allogeneic products applies to EVs remains unknown. Studies over the past decade have also shown that phagocytes can develop immunological memory to foreign biomolecules, which is thought to require an initial stimulation by the adaptive immune system.
Although autologous EVs face less immune clearance, they encounter considerable challenges, such as high production costs and labor-intensive operations. Additionally, the therapeutic and drug delivery effects of autologous EVs may vary greatly due to the heterogeneity of EVs between individuals and across healthy/disease conditions. Screening for the best allogeneic donors and pooling donor EVs can reduce batch-to-batch variability. Therefore, allogeneic EVs have the potential to reduce manufacturing costs, improve batch consistency, and ensure efficacy. This article critically evaluates the potential immunogenicity of EVs and discusses emerging strategies to mitigate adverse immune recognition of EVs for next-generation therapeutics and drug delivery systems.
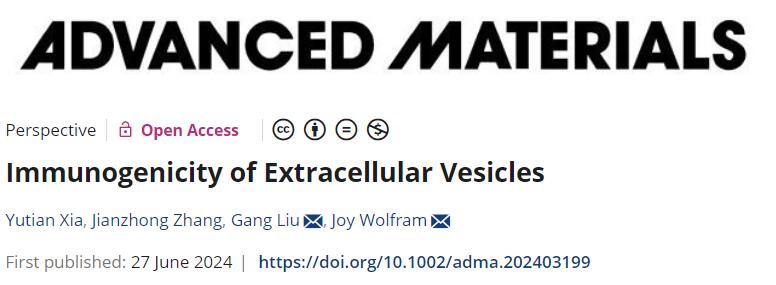
The immunogenicity of EVs may be affected by multiple factors, including origin (source and biogenesis), size, endogenous/exogenous content, production and storage methods, dose, infusion rate, and biomolecular coron.
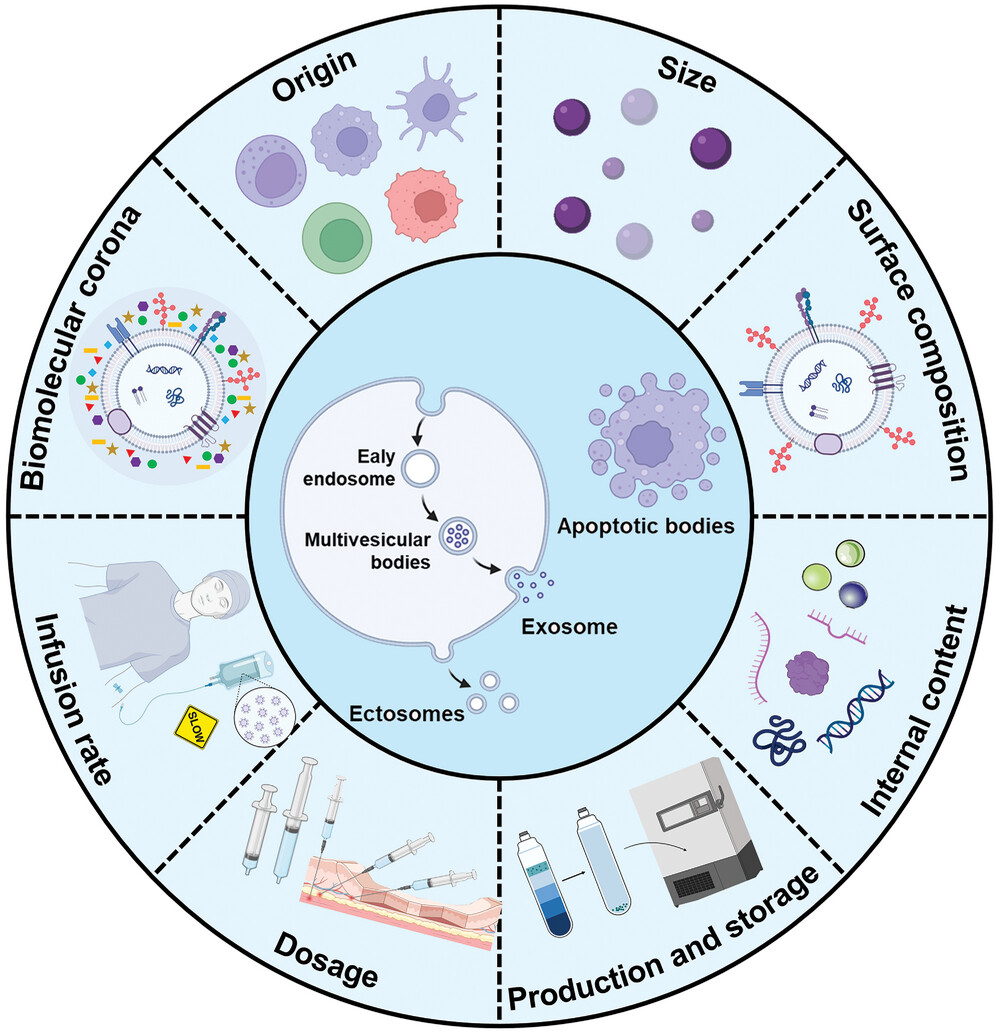 Examples of the biogenesis of EVs and factors that may affect the immunogenicity of EVs
Examples of the biogenesis of EVs and factors that may affect the immunogenicity of EVs
1.1 Sources of EVs
In general, transplanted cells can only successfully evade immune recognition if they are fully genetically and epigenetically identical to the recipient. However, achieving this consistency is often challenging. Stem cells (such as embryonic stem cells, mesenchymal stromal cells (MSCs), and adipose-derived stem cells) have low immunogenicity and are able to release EVs that play a key role in intercellular communication and immune suppression. Cancer cells also evade immune surveillance, and their derived EVs can maintain immune evasion properties and promote the formation of an immunosuppressive environment. Understanding these interactions is important for designing therapeutic EVs with immune evasion properties.
1.2 Size of EVs
EVs vary widely in size, with different types of EVs displaying distinct size characteristics. Exosomes (30-200 nm), ectosomes (0.1-1 µm), and apoptotic bodies (1-5 µm) overlap in size, affecting their interactions with immune cells. Small EVs show better delivery in tumors and inflammatory tissues, while larger EVs exhibit different accumulation patterns in specific organs. The balance between size, clearance rate, and target cell uptake rate is key to developing effective EV therapeutics and drug delivery systems.
1.3 Surface Composition of EVs
Specific surface components can either camouflage EVs from the immune system or accelerate their recognition. EV surface proteins (such as tetraspanins, lectins, and integrins) play a key role in immune cell interactions through ligand-receptor mechanisms. Advances in engineering technologies have made it possible to couple or express a variety of “don’t eat me” signaling molecules (such as CD47, CD55, CD59, and CD200) on the surface of EV membranes to overcome phagocytic clearance. The most widely studied “don’t eat me” receptor is the signal regulatory protein α (SIRPα) on macrophages, which specifically recognizes the widely expressed transmembrane protein CD47. CD47 binds to SIRPα on macrophages and inhibits macrophage-mediated phagocytosis. CD47 is present on various EVs and reduces clearance by the mononuclear phagocyte system.
In addition, efficient delivery of EVs also requires evading recognition by other immune cells, including natural killer (NK) cells and T cells. MHC-I molecules are widely expressed on the surface of all nucleated cells and play a key role in presenting endogenous antigens. During the production process of EVs, MHC-I molecules are inevitably integrated into the vesicle membrane. Gene editing technology enables selective targeting and knock out of the beta2-microglobulin (B2M) gene, thereby effectively reducing the expression of MHC-I molecules and making the immunogenicity of such EVs significantly lower compared to EVs containing intact MHC-I molecules.
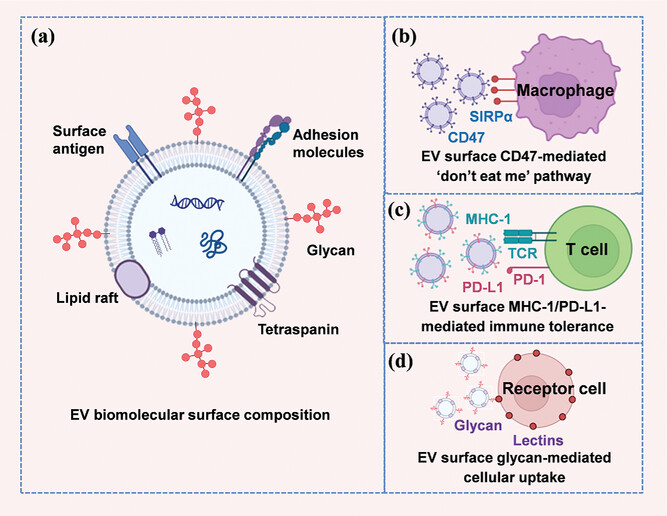 EV biomolecular surface composition and factors affecting immunogenicity. a) EV biomolecular surface composition. b) EV surface CD47-mediated “don’t eat me” pathway. c) EV surface MHC-1/PD-L1-mediated immune tolerance. d) EV surface glycoconjugate-mediated receptor cell uptake.
EV biomolecular surface composition and factors affecting immunogenicity. a) EV biomolecular surface composition. b) EV surface CD47-mediated “don’t eat me” pathway. c) EV surface MHC-1/PD-L1-mediated immune tolerance. d) EV surface glycoconjugate-mediated receptor cell uptake.
Non-classical MHC-I molecules (such as MHC-E, MHC-F, and MHC-G) can inhibit NK cell activity. In the context of cancer cells, abnormal expression of MHC-G allows the release of EVs containing MHC-G, thereby promoting the establishment of immune tolerance in the host. Immune checkpoint molecules such as programmed death ligand 1 (PD-L1) can inhibit T lymphocyte function. Increasing the expression of PD-L1 on the surface of MSC-derived EVs exhibits immunosuppressive effects on T cells, macrophages, and dendritic cells.
Sugars and lipids on the EV membrane are also important signaling molecules that affect EV immunogenicity. The outer membrane surface of EVs is rich in sugars, and its glycoconjugate spectrum plays a key role in cellular uptake and biodistribution. Lipids also play an active role in intercellular communication and immune regulation. For example, lipids in cancer cell-derived EVs can produce immunomodulatory effects, including metabolic remodeling and regulation of immune cell signaling pathways.
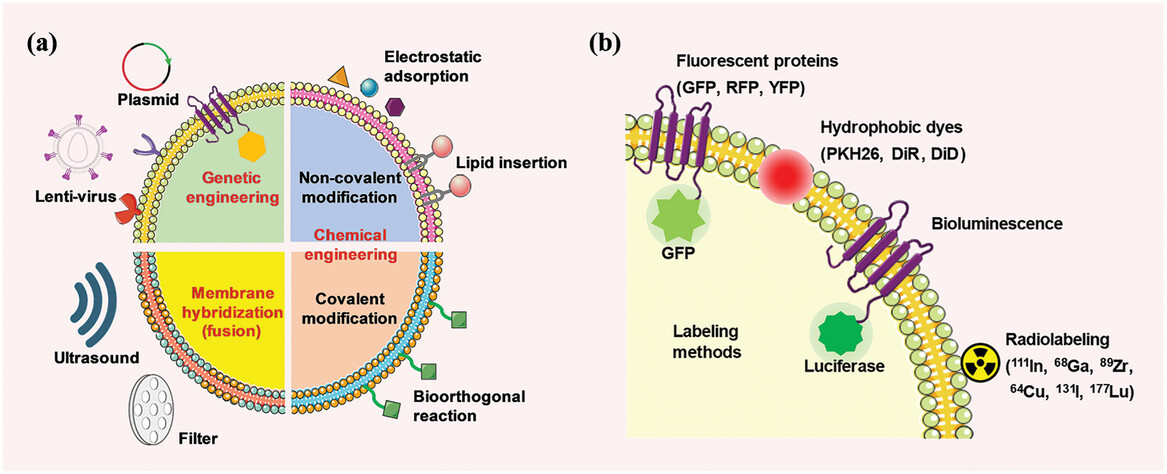 EV surface engineering methods and labeling strategies. a) EV engineering methods, including genetic engineering, chemical engineering, and membrane hybridization. b) EV labeling strategies, including fluorescent, luminescent, and radioactive labeling.
EV surface engineering methods and labeling strategies. a) EV engineering methods, including genetic engineering, chemical engineering, and membrane hybridization. b) EV labeling strategies, including fluorescent, luminescent, and radioactive labeling.
Choosing the appropriate engineering method is crucial for achieving optimal expression of biomolecules on the surface of EVs. Common engineering techniques include genetic engineering, chemical engineering, and membrane hybridization. Genetic engineering involves transfecting recombinant DNA into host cells through expression vectors (such as plasmids or lentiviruses) to achieve the expression of the desired protein. Chemical engineering techniques can be divided into non-covalent and covalent modifications. EV membrane hybridization (fusion) combines EV cell membranes with artificial membranes through methods such as physical extrusion, ultrasound, and electroporation. Common EV labeling methods include fluorescence, luminescence, and radioactive labeling, but these methods may affect the function and immunogenicity of EVs.
1.4 Internal Components of EVs
In addition to the surface components of EVs, endogenous and exogenous internal cargoes can also show immunomodulatory effects. For example, the circular RNA circNEIL3, which is highly expressed in brain gliomas, is encapsulated in EVs and engulfed by macrophages, resulting in an immunosuppressive phenotype. Various EV engineering methods can be used to load exogenous cargoes inside EVs, but these methods may affect the properties and immunogenicity of the EVs. Indirect loading involves exposing the source cells to the cargo, though loading efficiency is limited. Direct methods for loading internal cargo include ultrasound, extrusion, pore formers, and repeated freeze-thaw cycles, which can temporarily create openings in the membrane. However, studies have shown that ultrasound and extrusion may reduce the activity of enzymes carried by EVs, potentially causing damage or loss of endogenous biomolecules.
Besides, source cells can be genetically engineered to express proteins or RNA and load them into the interior of EVs. Compared to physical and chemical loading methods, genetic engineering has less effect on the structural integrity of EVs. Loading efficiency can be improved by fusing the desired biomolecules to internal proteins of EVs. Genetic engineering techniques have also been used to endow EVs with immunomodulatory properties. For example, genetically engineered EVs loaded with CC16 protein have demonstrated anti-inflammatory effects by regulating macrophages and neutrophils in an acute pneumonia mouse model.
1.5 Production and Storage of EVs
Production and storage methods can result in varying degrees of EV damage, thus affecting their immunogenicity. For example, the state of the source cells may influence the immunogenicity of EVs. Cellular stress can release EVs containing specific immunomodulatory mediators through stress-induced pathways. Separation methods (such as ultracentrifugation and dead-end filtration) may cause EV aggregation, membrane damage, and biomolecule damage, which could trigger immune responses. Exposed intracellular biomolecules from cells can trigger immune responses, but it is unclear whether exposed internal EV cargoes have a similar effect. Tangential flow filtration can be used as a gentle EV isolation method (controlled shear rate) to reduce EV damage and, thus, mitigate its immunogenicity.
Engineering EVs to carry targeting, imaging, and therapeutic agents may also cause EV damage, and these components could trigger immune responses. The extent of contaminants in EV samples may also affect immune responses. For example, hyaluronan, whose effects on the immune system depend on molecular weight, was recently found to be a contaminant in EV samples, with levels varying depending on the EV isolation method. Ultracentrifugation and precipitation kits result in the co-precipitation of numerous protein contaminants, while all size-based EV isolation methods retain protein aggregates, which may affect dosing accuracy or misattribute immunomodulatory effects to EVs. Density gradient ultracentrifugation can be used to increase the purity of EV samples.
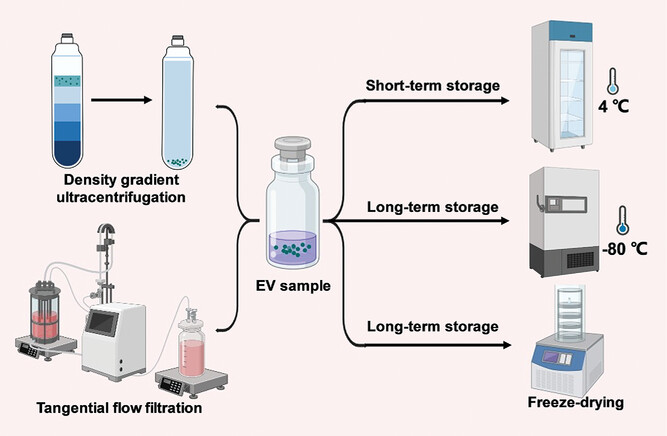 Examples of EV production and storage methods. Tangential flow filtration causes less EV damage and aggregation than ultracentrifugation, which may mitigate immune recognition. Density gradient ultracentrifugation yields higher purity EVs than ultracentrifugation, which may affect their immunogenicity. Storage methods may affect EV integrity and potential immunogenicity.
Examples of EV production and storage methods. Tangential flow filtration causes less EV damage and aggregation than ultracentrifugation, which may mitigate immune recognition. Density gradient ultracentrifugation yields higher purity EVs than ultracentrifugation, which may affect their immunogenicity. Storage methods may affect EV integrity and potential immunogenicity.
In clinical practice, immediate use of freshly prepared EVs is often not feasible, making storage a key consideration for the clinical translation of EV therapeutics and drug delivery systems. Typically, EVs can be stored at 4°C for a short period (<48 hours), while long-term storage requires −80°C conditions. However, the potency of EVs generally decreases after storage. Repeated freeze-thaw cycles and prolonged storage can cause EV fusion or aggregation, increase particle size and reduce potency. The lack of cryoprotectants can affect the integrity of EVs, thereby influencing their immunogenicity.
1.6 EV Dosage
The dose and route of administration of EVs are key factors affecting their immunogenicity. Different routes of administration (e.g., intravenous, intraperitoneal, and subcutaneous) result in different biodistribution and immune cell exposure patterns. In mouse models, intravenously injected EVs rapidly accumulate in the liver and spleen and are also distributed in the lungs and kidneys. Intraperitoneally injected EVs are better absorbed in adipose tissue than intravenously injected EVs, while subcutaneous and intraperitoneal injections increase EV absorption in the gastrointestinal tract and pancreas.
1.7 EV Infusion Rate
Intravenously infused therapeutics and drug delivery systems face the challenge of complement-mediated infusion reactions in clinical translation. For example, the interaction of anti-polyethylene glycol (PEG) antibodies with PEGylated nanoparticles may trigger a complement response, leading to anaphylactic shock. Various therapeutic approaches can cause infusion reactions, which usually occur within minutes of the start of infusion. Premedication and reduced infusion rates can alleviate such reactions. Although EVs may also experience infusion rate reactions, there is currently a lack of evidence as the field is in early clinical development and relies primarily on rodent data.
1.8 The Biomolecular Canopy of EVs
Synthetic nanoparticles and EVs are surrounded by a layer of adsorbed biomolecules in biological environments, known as the biomolecular corona. The composition of the corona affects the biological identity of EVs, including immune recognition and clearance. Circulating opsonins (such as complement proteins, immunoglobulins, and coagulation factors) play an important role in mediating the uptake of nanoparticles and EVs by innate immune cells. Complement protein H is abundant in hepatocellular carcinoma EVs, inhibiting the activation of other complement proteins and promoting tumor progression. Reducing the cholesterol content of EV mimics can decrease complement adsorption. Lipoproteins on EVs also affect their interactions with immune cells. Nucleic acids (DNA, RNA) are important components of the EV biomolecular corona and affect immune regulation. Modifying the EV surface can change the corona composition and immune recognition. Removal of the biomolecular corona in donor fluids may affect the immunogenicity of EVs. Future studies should aim to identify EV engineering strategies that help form an ideal biomolecular corona.
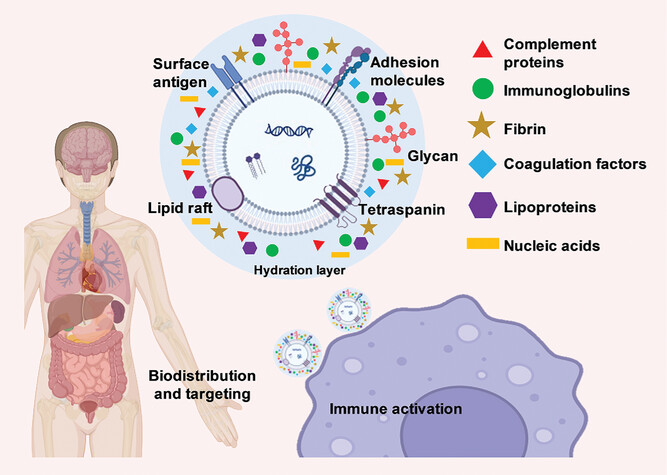 The composition of EV biomolecular surfaces and coronas and their impact on immunity, tissue targeting, and biodistribution
The composition of EV biomolecular surfaces and coronas and their impact on immunity, tissue targeting, and biodistribution
2 Conclusion and Future Outlook
The rapidly evolving field of EV therapeutics and drug delivery systems requires a thorough understanding of undesirable immunogenicity, which is critical for developing safe and efficient clinical products. These efforts necessitate interdisciplinary collaborations in immunology, cell biology, and nanomedicine. Although several preclinical and early clinical trials on EVs have demonstrated a remarkable lack of immunotoxicity, immune clearance remains poorly explored, especially in large animal models. Factors contributing to EV immunogenicity include source, size, surface composition, internal contents, production/storage methods, dose, infusion rate, and biomolecular corona. Understanding the impact of these factors on EV immunogenicity has the potential to inform the future development of optimal EV treatments and drug delivery systems. Various strategies to modulate EV immune recognition and clearance, including the addition and removal of EV surface components, have been explored. Future mitigation strategies may rely on improved EV production and storage methods combined with engineered immune evasion surfaces. Several biomolecules on the surface of EVs have been identified to possess either immunostimulatory or immune evasion properties. In the future, omics technologies (proteomics, glycomics, lipidomics, transcriptomics, and metabolomics), combined with functional studies, will be crucial for comprehensively identifying and implementing immune-evading EV surfaces while retaining specific targeting capabilities.
Reference:
Xia, Yutian et al. “Immunogenicity of Extracellular Vesicles.” Advanced materials (Deerfield Beach, Fla.), e2403199. 27 Jun. 2024, doi:10.1002/adma.202403199
Related Services:
