Extracellular vesicles (EVs), including exosomes, encapsulate DNA, RNA, and proteins within a phospholipid bilayer. These vesicles deliver their lumenal cargo to the cytoplasm of recipient cells, influencing immune cell function. This impact is partly due to the intersection of EV biogenesis with antigen processing and presentation pathways. EVs derived from activated immune cells can increase the frequency of immune synapses on recipient cells, regulating both local and systemic immunity in various conditions, such as inflammation, autoimmunity, organ fibrosis, cancer, and infection. Both natural and engineered EVs have demonstrated the ability to modulate innate and adaptive immunity and are currently being explored in clinical trials. EVs may be integral to a well-functioning immune system and hold promise as immunotherapeutic agents. Recently, Raghu Kalluri, a leading expert on exosomes, published a review in the journal Immunity, summarizing the biology and function of extracellular vesicles in the immune system.

Early functional studies suggested that EVs, containing DNA, RNA, proteins, and lipids, might play a role in antigen presentation and adaptive immune responses. Such initial observations opened up an emerging field of research focused on the role of EVs in immune responses and overall immunity. The heterogeneous nature of EVs, their biogenesis, and their putative roles have become a central focus in multiple biomedical fields as researchers work to elucidate their significance in both physiological and pathological responses. All prokaryotic and eukaryotic cells secrete EVs that participate in complex biological signaling networks within recipient cells. The surface ligands and receptors on EVs reflect their cellular origin, with many cellular components being found within EVs. Some components are more commonly found in EVs and exosomes—a subtype of EVs originating from the endocytic pathway and typically ranging in size from 40 to approximately 180 nm.
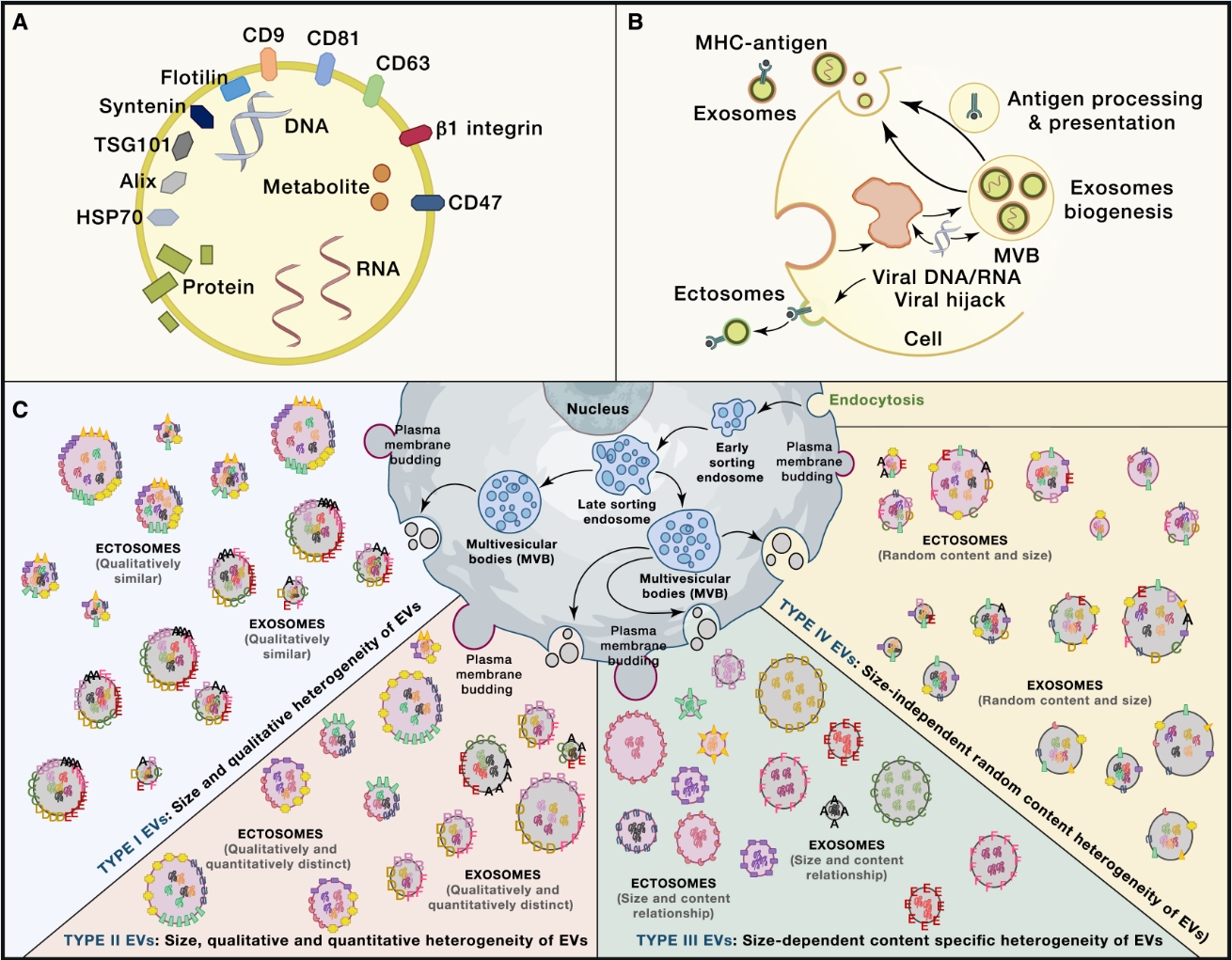 EV structure, biogenesis, and heterogeneity, relevant to antigen processing and presentation
EV structure, biogenesis, and heterogeneity, relevant to antigen processing and presentation
The biogenesis of EVs and exosomes may intersect with multiple pathways that regulate their content composition. EVs exhibit heterogeneity in their biogenesis, size, content, and structure.
The role of EVs in immunity: from early discoveries of antigen presentation to inducers of complex intracellular signaling networks
EVs have important functions in antigen presentation and adaptive immune responses, extending beyond their initial characterization as vehicles shedding membrane proteins in reticulocyte maturation. EVs released from dendritic cells (DCs) loaded with tumor peptides can directly stimulate T cells through MHC class II peptide complexes and exhibit antitumor activity in mice. This demonstrates the role of EVs in cross-priming, which stimulates the generation of activated CD8+ T cells from naive CD8+ T cells. EVs can also facilitate the exchange of MHC class II antigen complexes between DCs to activate T cells, as well as influence the intercellular programming of DCs by mediating the exchange of miRNA between cells, highlighting their complexity in signaling. EVs derived from DCs and B cells can stimulate T cell proliferation and cytokine production in vitro, laying the foundation for the potential application of EVs as vaccines by directly or indirectly loading them with antigenic peptides. In vivo, EV exchange between DCs may enhance T cell activation during transplant rejection. Additionally, small DC-derived EVs may deliver different polarization signals to T cells depending on the maturation state of the DCs, compared with larger ones, emphasizing the functional heterogeneity of EVs and the impact of cell state on their function. Not only do DCs secrete functional EVs, but their differentiation in vitro is also influenced by cancer cell-derived EVs, underscoring the dynamic and complex regulatory role of EVs in immune responses. In recent years, research has unveiled the role of EVs in infectious diseases (such as COVID-19), microbiota regulation, aberrant immune responses, and cancer development, driving the development of engineered EVs as immunotherapeutic agents. Given the potential role of EVs in antigen presentation, especially in T cell cross-priming, EVs may play an important role in the development of antiviral vaccines and anticancer strategies. Crucially, the roles of EVs as immunomodulators are functionally diverse and reflect the dynamic contributions of their microenvironment and cellular origin. For example, EVs are involved in T cell cross-priming and cross-tolerance, can either enhance or limit viral immunity, promote or inhibit anti-tumor immune responses, regulate or dysregulate the microbiota, and exacerbate or reduce inflammatory responses. The bimodal functions of EVs represent their diverse cellular origins and dynamic microenvironments, suggesting their pivotal role in the multidirectional calibration of immune signaling.
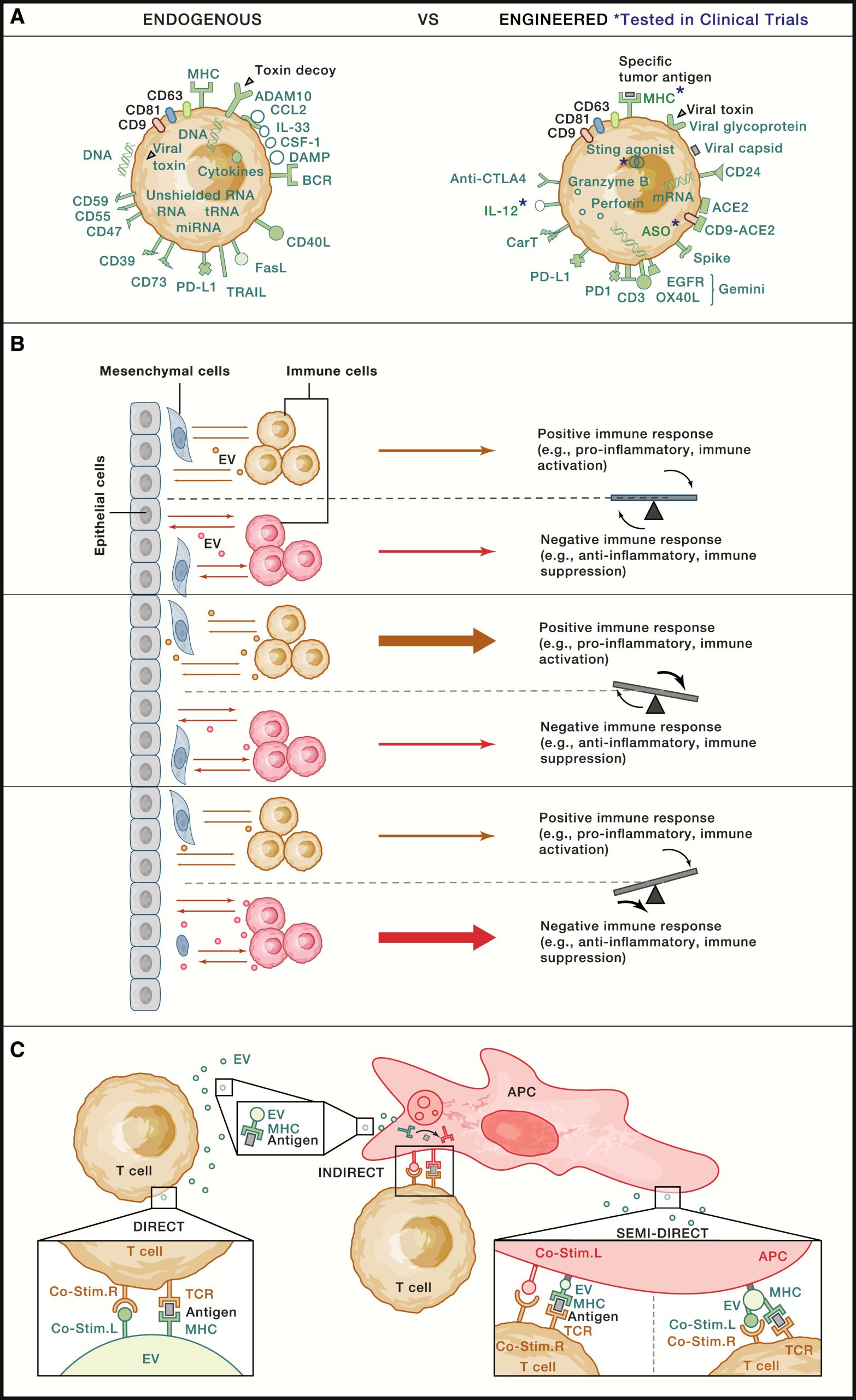 EVs from different cell types within the ecosystem are responsible for coordinating antigen presentation and regulating immune responses, thereby influencing tissue damage and disease states.
EVs from different cell types within the ecosystem are responsible for coordinating antigen presentation and regulating immune responses, thereby influencing tissue damage and disease states.
EVs can modulate the immune system: contextual production of EVs shapes bimodal immune responses
EVs are generated by all immune and tissue-resident cells, playing key roles in influencing multiple biological pathways associated with tissue injury, inflammation, autoimmunity, infection, and cancer. In different pathological contexts, EVs produced by fibroblasts, vascular endothelial cells, lymphatic endothelial cells, B cells, T cells, natural killer (NK) cells, dendritic cells (DCs), macrophages, and neutrophils may work in a coordinated manner to provide both positive and negative signals.
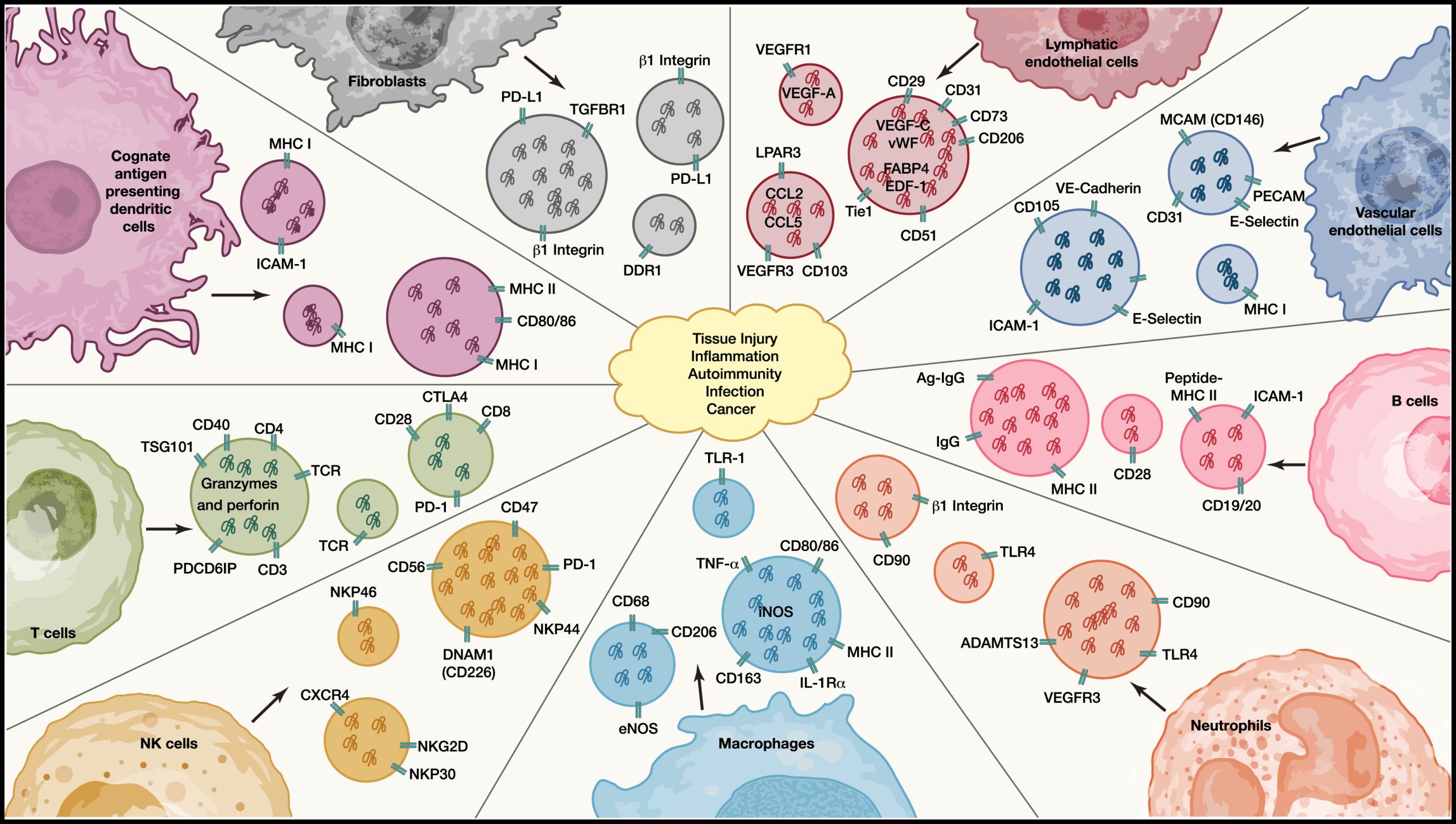 Many different cell types, including immune cells, are involved in generating EVs that reflect their biological phenotype and provide positive and negative signals to influence tissue fate
Many different cell types, including immune cells, are involved in generating EVs that reflect their biological phenotype and provide positive and negative signals to influence tissue fate
The environment of EVs influences tissue homeostasis and diverse immune signaling to regulate tissue injury, inflammation, autoimmunity, infection, and cancer. EVs may functionally protect tissues or promote disease depending on the context, reflecting the cellular and molecular signals received by their cells of origin. The ultimate outcome of EVs in tissues may depend on how these diverse activities are balanced.
To successfully initiate and execute an effective adaptive immune response, coordinated interactions between dendritic cells (DCs), T cells, B cells, and antigenic targets need to occur appropriately and in sequence. Different types of leukocytes must interact in lymph nodes (LNs), secondary lymphoid organs (SLOs), and peripheral tissues in response to a single antigenic stimulus. All participating cells must generally be in the same location so they can coordinate their interactions and form effective immune synapses. For example, successful activation of naive T cells by antigen-presenting DCs may require several specific immune synapses, a requirement that may not be met by incidental contact between the two cells alone. Therefore, it is conceivable that EVs and exosomes serve as important alternatives to promote the rapid formation of multiple additional immune synapses to support an orderly immune response. Conversely, the spatiotemporal organization within SLOs is essential not only to initiate immunity but also to tailor the type of response generated. This EV-mediated immune modulation (EMIM) may be an additional branch of the immune system for the rapid and systemic dissemination of immunity.
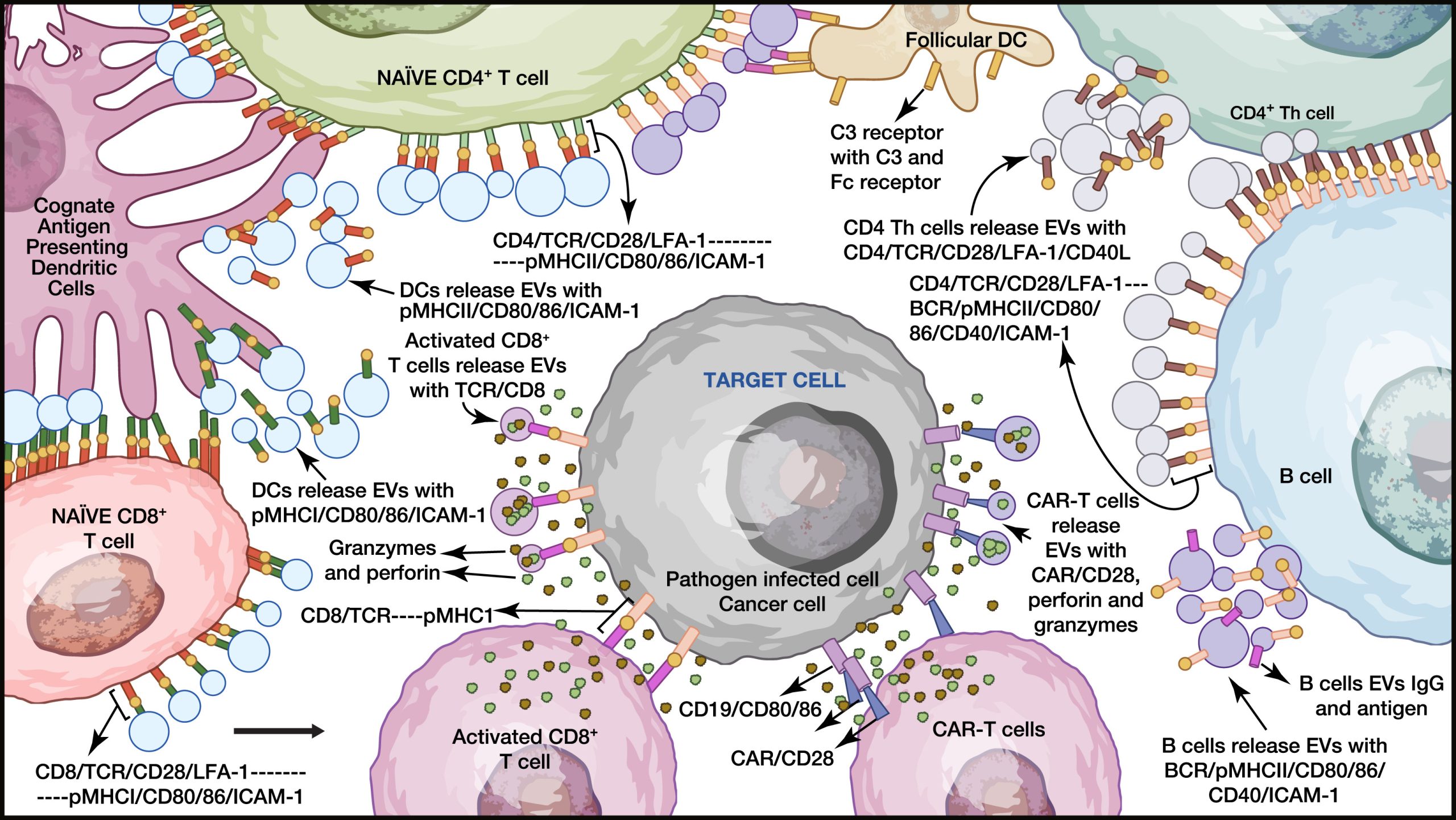 Potential contribution of EVs in regulating systemic immune responses
Potential contribution of EVs in regulating systemic immune responses
Recent evidence suggests that EVs may represent an underappreciated branch of the immune system, capable of rapidly amplifying immune signals systemically without requiring cell-to-cell proximity and direct interactions. EVs may fulfille these requirements, as shown in this schematic. EVs released by DCs, B cells, CD4+ T cells, and CD8+ T cells can mediate interactions that promote immunity and weaken target cells. This system, regulated by EVs, is termed “EV-mediated immunomodulation” (EMIM) and may represent a new branch of the immune response.
In addition, the review introduces the following points:
- EVs regulate the metabolic program of immune cells.
- EVs can be exploited by pathogens but can also be produced to defend against them.
- EVs serve as viral baits and have potential use in vaccine development.
- EVs regulate the microbiota.
- EVs are involved in abnormal immune responses, such as chronic inflammation and autoimmunity.
- EVs play a role in tumor immunotherapy
The role of EVs in orchestrating complex immune responses is gaining increasing attention, with numerous studies demonstrating that their cargo selection and effects on recipient cells are dynamic and influenced by their microenvironment. The dual functions of EVs in inflammatory responses, immune cell signaling, and immunity are primarily derived from in vitro studies or observations of exogenous EVs. These observations must be validated using appropriate and relevant in vivo models to determine their rate-limiting functions in both physiological and pathological contexts. Questions regarding the dosage of EVs in immune responses complicate the physiological relevance of many studies, especially when considering the limitations of current isolation methods. Nonetheless, the consistent observation of EV effects on the regulation of innate and adaptive immune responses supports their function as regulators of the immune system. Future studies should consider using relevant genetic models with in vivo generation of immunomodulatory active EVs to study them at natural and physiological concentrations.
EVs may act as “first responders” in response to tissue damage, serving as sensors, initiators, and controllers of immune responses. The context-dependency and dynamic evolution of their contents make EVs unique immunomodulatory components in the body, capable of refining the direction of immune responses—whether enhancing or suppressing them. With the application of optimized isolation techniques with single vesicle resolution, the more precise effects of EVs on immune system cells will be recognized.
Despite the challenges of studying natural EVs, the therapeutic potential of engineered EVs remains attractive, as they can be designed to either suppress or stimulate immune responses. Clinical studies of EVs involve challenges such as the scalability and stability of clinical products, the selection of appropriate cell sources, and the impact of engineering changes on safety. However, EVs may become key players in immune responses and regulation in both health and disease, and they hold significant therapeutic potential.
Reference:
Kalluri, Raghu. “The biology and function of extracellular vesicles in immune response and immunity.” Immunity vol. 57,8 (2024): 1752-1768. doi:10.1016/j.immuni.2024.07.009
Related Services:
Exosome Lipidomics & Metabolomics Services
