Cancer stands as one of the leading causes of death globally, necessitating a deeper understanding of its progression factors. Exosomes, with an average size of 100 nm, serve as carriers of proteins, lipids, and nucleic acids. In a recent review published by researchers from Iran’s Islamic Azad University in the Journal of Hematology & Oncology, the mechanisms and functions of exosomes in cancer progression and treatment, particularly their involvement in tumor microenvironment remodeling, were comprehensively explored.
Cancer poses a significant threat to life and health worldwide, ranking as the second leading cause of death following cardiovascular disease. Cancer cells possess distinct traits including high proliferation rates, self-renewal capacity, characteristics resembling cancer stem cells (CSCs), metastatic potential, and the ability to develop drug resistance by switching between molecular pathways. Leveraging these properties, researchers have devised innovative therapies, including nucleic acid drugs and anticancer agents, to target and impede cancer cell progression. In addition, advancements like nanoparticle utilization have facilitated precise delivery of therapeutic agents to cancer cells.
Recent focus has shifted towards understanding the role of extracellular vesicles (EVs) in cancer. Originating from cell membranes, EVs encompass microvesicles or nanovesicles and are secreted by all prokaryotic and eukaryotic cells in a conserved manner across evolution. Initially perceived as cellular debris or byproducts of damage, further investigations have revealed their vital biological functions and significance as cellular components. EVs categorized based on size and source, include exosomes, particles, shed vesicles, apoptotic bodies, tolerosomes, proteasomes, and preproteasomes. Two distinct mechanisms govern EV formation: direct budding from the cell membrane and generation during multivesicular body exocytosis within the endocytic system. Engaging in various cellular functions, EVs play pivotal roles in pathological conditions, facilitating molecular transfer between cells and serving as crucial mediators of cellular communication. Hence, they hold unique potential in disease diagnosis and treatment, particularly in the realm of cancer.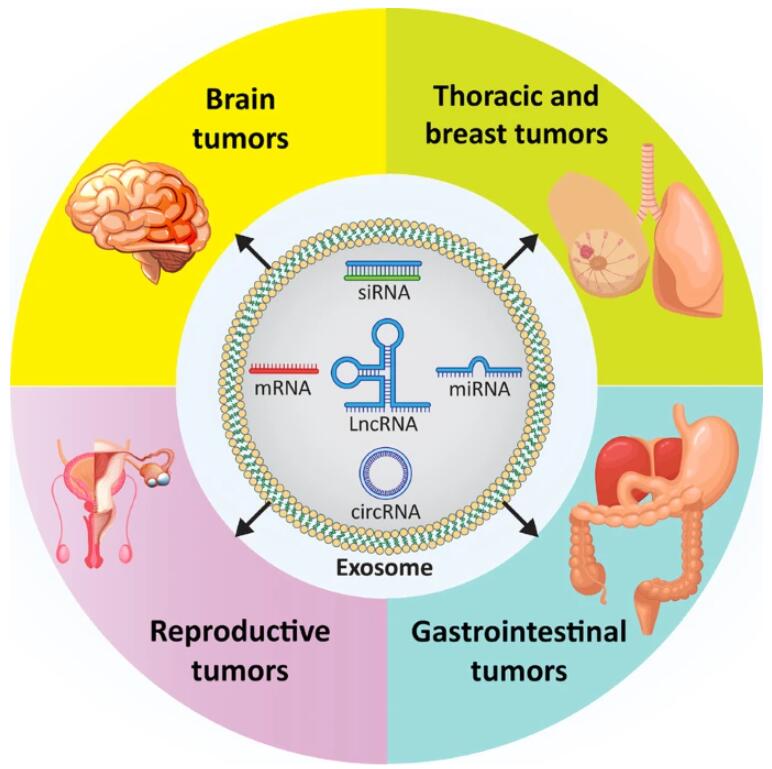
In this review, researchers focus on the role of exosomes in cancer. It begins with an overview of the discovery of exosomes, their composition, and their biogenesis pathways, crucial for understanding these structures. The researchers then delve into describing the role of exosomes in tumor microenvironment (TME) remodeling and how they influence various cancer hallmarks, including proliferation, migration, and treatment response.Subsequently, the article discusses how exosomal noncoding RNAs (ncRNAs) affect cancer cell progression, as well as explores exosomes and key molecular signaling pathways regulating cancer progression. Finally, insights into tumor-derived exosomes and the clinical applications of exosomes relevant to the treatment of cancer patients are provided.
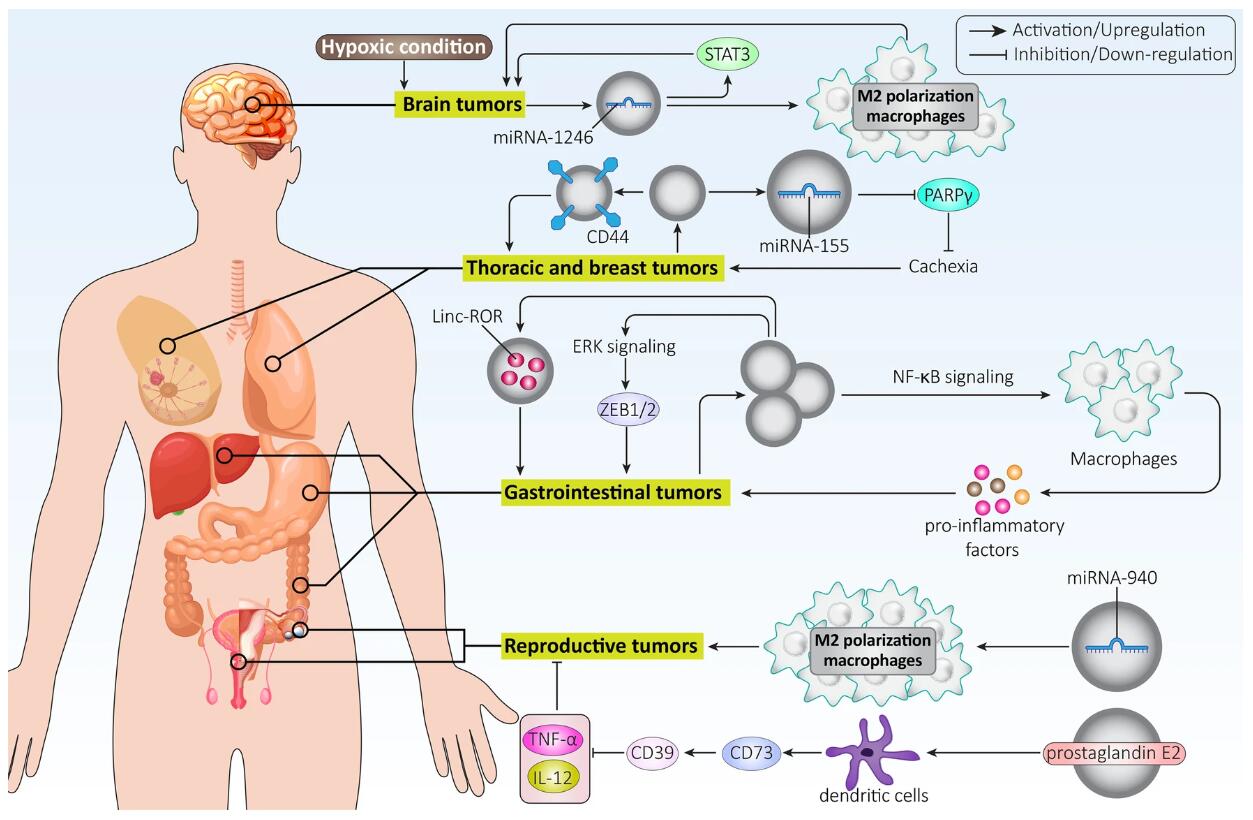
First, the review explores the impact of EVs on tumor growth and invasion. When exosomes contain tumor-promoting substances, they can promote cell cycle progression and glycolysis, inhibit apoptosis, and their role in autophagy is more complex. Therefore, further experiments are recommended to reveal the interplay between apoptosis and autophagy in cancer cells affected by exosomes. Similar to proliferation, exosome cargo determines the function of these structures in therapeutic migration and invasion. EMT and MMPs are strongly influenced by exosomes in regulating cancer progression. However, most studies have focused on the EMT mechanism, and due to the crucial role of MMPs, it is suggested to unveil the signaling network affected by exosomes in targeting MMPs and regulating cancer metastasis. Cancer cell proliferation and invasion rates determine the response to treatment. If cancer cells have a high ability to migrate and grow, they may become resistant to treatment. Therefore, targeting exosomes may modulate the proliferation and invasion of cancer cells and predict their response to treatment. The aggressive behavior of cancer cells mainly depends on interactions in the TME, such as macrophage polarization mediated by exosomes.
Secondly, the review addresses the prediction of EV response to tumor treatment, a clinical concern. Exosomes can reduce the sensitivity of cancer cells to chemotherapy-mediated apoptosis. As exosomes have the potential to transfer various genes, they can influence the progression of cancer cells and determine their response to treatment. In addition to drug resistance, exosomes may also be involved in triggering radiotherapy resistance. In addition, exosomes can induce immune cell exhaustion, reduce T cell cytotoxicity, and mediate immune evasion. By triggering chronic inflammation, exosomes promote cancer progression.
Thirdly, the review highlights the internalization mechanism of EVs, a focus of research. Exosomes are able to bind to receptors on the cell surface and can be internalized by binding to integrins, tetraspanins, and intercellular adhesion molecules. The internalization mechanisms of EVs include clathrin- and caveolin-mediated endocytosis, lipid raft uptake, macropinocytosis, phagocytosis, and fusion with the plasma membrane. Therefore,elucidating the internalization methods of exosomes is crucial for their use as cargo transporters in cancer therapy, with subsequent functionalization improving their intracellular accumulation.
Fourthly, the review explores the research and application of EVs in drug delivery. Since exosomes can deliver various drugs, they have the potential to be used as delivery systems for anti-tumor drugs and as genetic tools in cancer treatment. Various anticancer agents, including plant-derived natural compounds such as triptolide and synthetic agents such as cisplatin, doxorubicin, and paclitaxel, can be transferred via exosomes for cancer treatment. siRNA and CRISPR/Cas9 are genetic tools embedded in exosomes for cancer treatment. Using exosomes to deliver therapeutic agents may lead to increased intracellular accumulation and improved therapeutic efficacy. Additionally, the surface of exosomes can be modified with ligands to increase their selectivity toward cancer cells.
Lastly, the review discusses the application of EVs in liquid biopsy and cancer treatment. Since exosomes can be isolated from patients’ serum, they are considered reliable biomarkers for the diagnosis and prognosis of cancer patients. Exosomes have been used as biomarkers in various experiments in cancer patients. In addition to diagnostics, exosomes are also being used to improve the accuracy of other testing methods for cancer patients, such as CT. There is currently a clinical trial underway using curcumin as an anti-cancer agent for the treatment of colon cancer, the results of which may provide new insights into the role of exosomes as drug delivery systems in clinical processes and their safety. Additionally, several clinical trials are currently underway on molecular profiling of exosomes, which may have a role in precision medicine in the near future.
Reference:
Paskeh MDA, Entezari M, Mirzaei S, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 2022;15(1):83. Published 2022 Jun 28. doi:10.1186/s13045-022-01305-4
Related Services:
Tumor Model Construction Service for Exosome Functional Research
Tumor Diagnosis-Applied Exosomes
Tumor Cells-Targeted Exosome Modification Service