Autophagy mediates organelle and protein transport, cell quality control, and metabolism. However, this mechanism also controls the secretion of extracellular vesicles (EVs) through unconventional pathways, such as endosome-related secretory routes. Tumor cells tend to release more EVs, and the impact of autophagy inhibitors on EV secretion is of significant interest in anti-tumor therapy. Recently, a review published in the journal Molecular Cancer discussed how different autophagy inhibitors influence EV secretion, highlighting the non-specific effects of these inhibitors and focusing on endosome-related secretory pathways.

Autophagy is a highly conserved evolutionary process vital for maintaining organelle and protein turnover, cellular quality control, and metabolism. Lysosomal-mediated autophagic degradation not only recycles cytoplasmic materials to provide nutrients and energy but also removes toxic protein aggregates and defective organelles. This recycling pathway also affects cell specialization and differentiation, protein trafficking, and unconventional secretory processes. There are three types of autophagy observed in mammalian cells: macroautophagy, microautophagy, and chaperone-mediated autophagy. During microautophagy, the lysosomal membrane directly partitions the autophagic cargo, while macroautophagyinvolves the formation of double-membrane structures called autophagosomes, which deliver the cargo to endosomes or lysosomes. Macroautophagy is also involved in the selective degradation of organelles during processes like mitophagy, nuclear autophagy, and exophagy. Chaperone-mediated autophagy targets the selective degradation of KFERQ-like motif proteins, which are delivered to lysosomes by the chaperone protein HSC70 and other auxiliary chaperones (such as CHIP, HOP, and HSP40). The internalization of cargo into lysosomes is controlled by the receptor lysosomal-associated membrane protein 2A (LAMP2A). In this review, the researchers primarily focused on macroautophagy.
Endocytosis is a cellular process that isolates materials from the external environment by engulfing them into vesicles, separating them from other cellular components. This process includes clathrin-dependent pathways as well as clathrin-independent pathways, such as phagocytosis, pinocytosis, raft-mediated endocytosis, and ARF6-dependent internalization. Like autophagy, endocytosis ultimately leads to lysosomal degradation, but in this case, the cargo is internalized from the plasma membrane rather than the cytoplasm. Once internalized, the cargo is sorted into highly dynamic compartments known as early endosomes (EEs), which are marked by specific adaptor proteins, effector proteins, and small Rab GTPases, including RAB4, RAB5, EEA1, VPS34, and SNAREs. EEs serve as major cellular sorting platforms, capable of maturing into endosomes that determine the fate of the cargo. The cargo in EEs can be recycled back to the plasma membrane via recycling endosomes, transported to or from the Golgi via the retrograde complex, or directed to lysosomes via multivesicular bodies (MVBs)/late endosomes. Autophagy and endocytosis pathways cooperate at various stages, mediating a range of molecular mechanisms.
Recent studies have revealed numerous interconnections between autophagy, exosomes/amphisomes, and EV exocytosis. For the release of exosomes or endosomes, several steps are required, including the biogenesis of intraluminal vesicles (ILVs) within MVBs, the transport of MVBs to autophagosomes or the plasma membrane, and the fusion of MVBs with the plasma membrane. These steps are influenced by components of the autophagy machinery, including various Rab GTPases such as RAB7, RAB11, RAB35, RAB27A, RAB27B, and VAMP7. RAB7 and RAB11 are also involved in autophagosome formation, with RAB7 playing a key role in autophagosome maturation. Therefore, autophagy can have either stimulatory or inhibitory effects on EV secretion, and these effects may be closely related to the cellular environment. This dual role of autophagy in cancer progression may account for its double-edged sword properties.In this review, we explore how different autophagy inhibitors affect EV secretion and summarize the non-specific effects of autophagy inhibitors, with a particular focus on the endosome-related secretion pathway. Modulating autophagy significantly affects not only the quantity of EVs but also their content, which could have implications for the pro- or anti-tumor effects of autophagy inhibitors in solid cancer therapies.
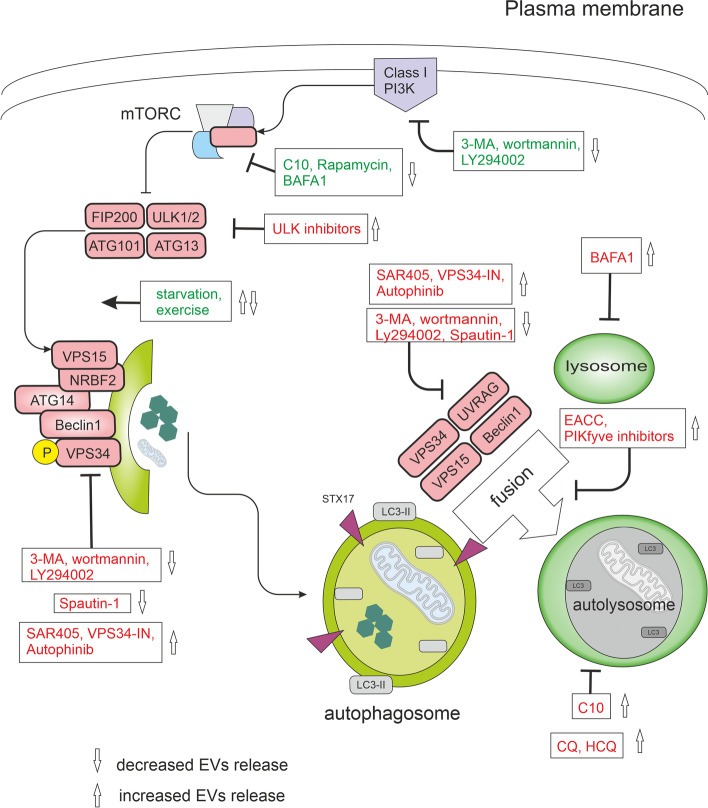 Autophagy regulators and their effects on EV release
Autophagy regulators and their effects on EV release
Under nutrient-rich conditions, mTORC1 blocks the ULK complex and autophagy. mTORC1 signaling can be directly inhibited by factors such as C10, rapamycin, exercise, or starvation. The ULK complex, when activated, stimulates the VPS34 complex, a class III phosphatidylinositol 3-phosphate kinase (PI3KC3). PI3K inhibitors, including 3-methyladenine (3-MA), wortmannin, and LY294002, effectively inhibit PI3K activity. In addition, VPS34 inhibitors, such as spautin-1, autophinib, SAR405, and VPS34-IN1, specifically target this pathway. Autophinib, an ATP-competitive inhibitor of VPS34, reduces the accumulation of lipidated protein LC3 on the autophagosomal membrane, thereby impeding autophagy progression. In the later stages of the autophagic process, fusion and degradation become critical. During the fusion stage, the mature autophagosome fuses with the lysosome to form an autophagolysosome. Inhibitors like PIKfyve inhibitors and EACC block the fusion of autophagosomes and lysosomes. Additionally, BAFA1 inhibits the acidification of autolysosomes by blocking V-ATPase, while chloroquine (CQ) and 3-hydroxychloroquine (HCQ) impair the maturation of autolysosomes. In this figure, activation steps are marked in green, while inhibition steps are marked in red.
Reference:
Raudenska, Martina et al. “Crosstalk between autophagy inhibitors and endosome-related secretory pathways: a challenge for autophagy-based treatment of solid cancers.” Molecular cancer vol. 20,1 140. 27 Oct. 2021, doi:10.1186/s12943-021-01423-6
Related Services:
