Extracellular vesicles (EVs) have emerged as a promising avenue for advanced drug delivery. One effective strategy for loading these EVs with desired cargo involves genetically fusing a target protein to a scaffold protein with exceptional EV sorting capabilities. To address the challenge of finding suitable scaffolding proteins, a recent article published in the journal Nature Communications has focused on identifying superior candidates. This research aims to enhance capacity of extracellular vesicles to transport specific cargo.

Extracellular vesicles are membrane-coated particles that various cell types release. These tiny vesicles play a crucial role in intercellular communication by transporting an array of macromolecules. Their innate tropism and protective characteristics shield their internal contents from rapid degradation. Combined with their favorable safety profile, extracellular vesicles have garnered significant attention as a potential next-generation therapeutic approach for a wide range of diseases.
The therapeutic potential of exosomes primarily hinges on their cargo. In the simplest scenario, extracellular vesicles naturally carry therapeutic molecules derived from their parent cells. For instance, mesenchymal stem cell-derived EVs have consistently exhibited the regenerative and immunomodulatory properties associated with their source cells. Alternatively, extracellular vesicles can be intentionally loaded with specific molecules through either endogenous or exogenous methods. Exogenous loading involves the manipulation of pre-isolated EVs through physical techniques like ultrasound, electroporation, or chemical covalent bonding. However, this method is generally limited to smaller payloads, including miRNAs and low molecular weight chemicals, and presents challenges related to RNA precipitation and potential physical damage or aggregation of EVs. Conversely, larger payloads, such as proteins, are typically loaded endogenously within the producer cells. In this approach, the cells are genetically modified to express the target protein fused with EV sorting proteins. This modification enhances the sorting of endogenous cargo proteins and can direct molecules to the surface or lumen of EVs. Notably, intraluminal loading prevents premature dissociation/degradation of cargo, making it the preferred method for molecules susceptible to degradation or those functioning within the recipient cell’s cytoplasm or nucleus. Studies have verified the feasibility of endogenous loading by incorporating or coating protein therapeutics (such as super-inhibitory IκB and receptor protein decoys) on EVs. Furthermore, this approach allows indirect loading of RNA therapeutics through the fusion of RNA-binding proteins to EV sorting proteins.
Although endogenous loading is a versatile strategy, it is essentially determined by the abundance of sorted proteins within the EV population. This determination not only hinges on the level of these proteins within each EV but also, more crucially, on their distribution across different EV subpopulations. In general, EV populations are complex and heterogeneous groups, which, until recently, have presented challenges in terms of characterization and physical separation into distinct subpopulations. This complexity has made the implementation of endogenous loading strategies more intricate. Fortunately, a breakthrough has been made with the discovery of 213 conserved proteins in EVs derived from 60 different cell types, as identified by the National Cancer Institute (NCI-60). These proteins now stand as potential candidates for EV sequencing. However, it’s worth noting that, to date, only a handful of proteins have been well characterized for their capacity to load into EVs. Most of these proteins belong to the tetraspanning superfamily and are characterized as multichannel transmembrane proteins, including well-known examples such as CD9, CD63, and CD81. Considering the inherent diversity of EVs, experiments involving the overexpression of the 63-GFP fusion protein have demonstrated that, in most cases, this resulted in 51% of particles being GFP-positive. Ongoing efforts have been made to identify alternative EV sorting candidates that show promise. These candidates include PTGFRN, BASP1, and TSPAN14. It’s important to note that these studies have primarily employed low-throughput, GFP-centered quantitative methods and have considered up to 14 candidate proteins in their investigations.
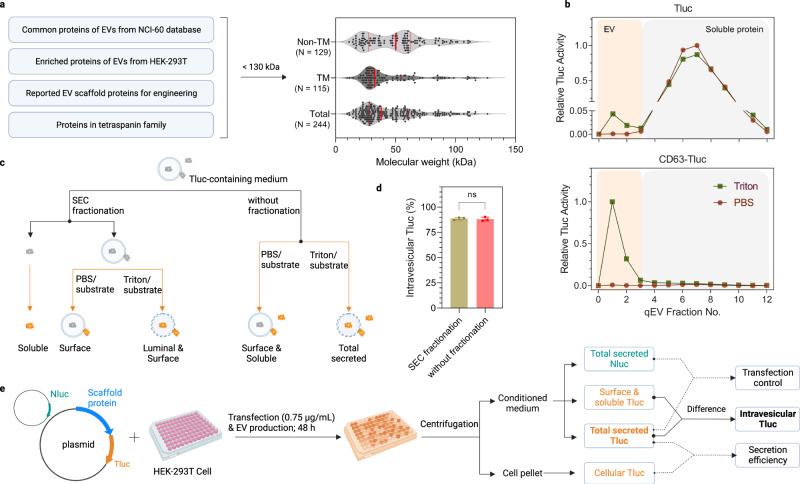 Bioluminescent screening protocol for quantifying luminal cargo proteins in EVs
Bioluminescent screening protocol for quantifying luminal cargo proteins in EVs
In this study, a large-scale comparative analysis was conducted involving 244 potential candidates to get a more comprehensive understanding and to potentially identify other EV sorting proteins. Throughout the research, TSPAN2, TSPAN3, and CD63 consistently emerged as efficient EV sorting proteins with robust intraluminal loading capabilities across various producer cell types. Furthermore, TSPAN2 and TSPAN3 engineered EVs exhibited not only effective uptake capabilities in vitro and in vivo but also demonstrated versatile delivery possibilities, including surface display and intraluminal cargo loading. Consequently, the authors believe that this discovery forms the basis for endogenous engineering approaches aimed at loading cargo into EVs for potential therapeutic applications.
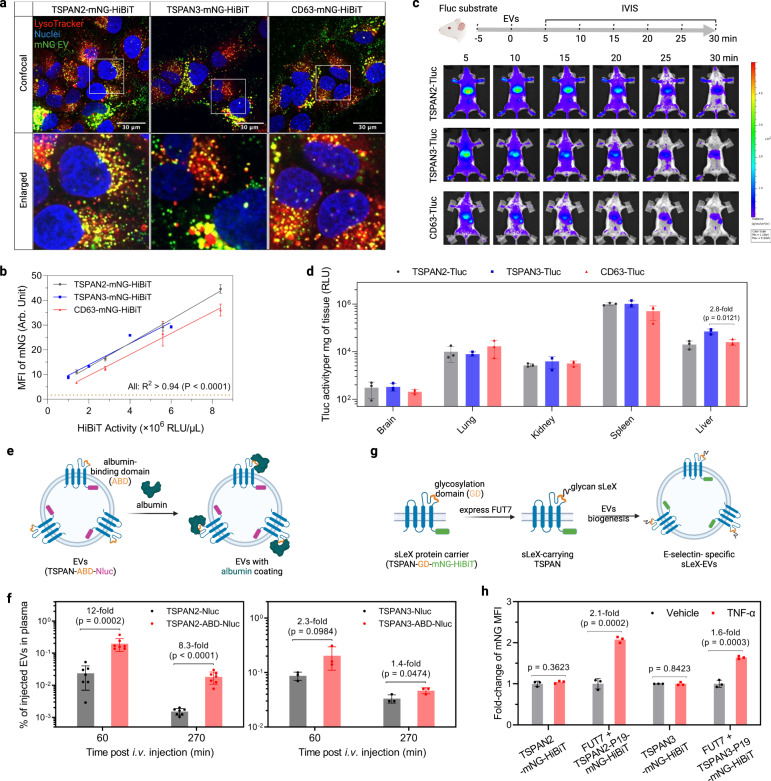 Biological activity of TSPAN2 and TSPAN3 engineered EVs
Biological activity of TSPAN2 and TSPAN3 engineered EVs
This study employed a straightforward and dependable assay capable of distinguishing between intravesicular and surface cargo proteins as well as non-vesicular proteins. The study screened 244 candidate proteins to identify 24 with consistent EV sorting capabilities across five producer cell types. TSPAN2 and TSPAN3 emerged as top candidates, surpassing the performance of the CD63 scaffold. Importantly, these engineered EVs showed potential as delivery vehicles in both cell culture and mice models, facilitating the efficient transfer of luminal cargo proteins and the surface display of different functional entities. The discovery of these scaffolds establishes a foundation for EV-based engineering.
References:
Zheng W, Rädler J, Sork H, Niu Z, Roudi S, Bost JP, Görgens A, Zhao Y, Mamand DR, Liang X, Wiklander OPB, Lehto T, Gupta D, Nordin JZ, El Andaloussi S. Identification of scaffold proteins for improved endogenous engineering of extracellular vesicles. Nat Commun. 2023 Aug 7;14(1):4734. doi: 10.1038/s41467-023-40453-0. PMID: 37550290; PMCID: PMC10406850.
Related Services:
