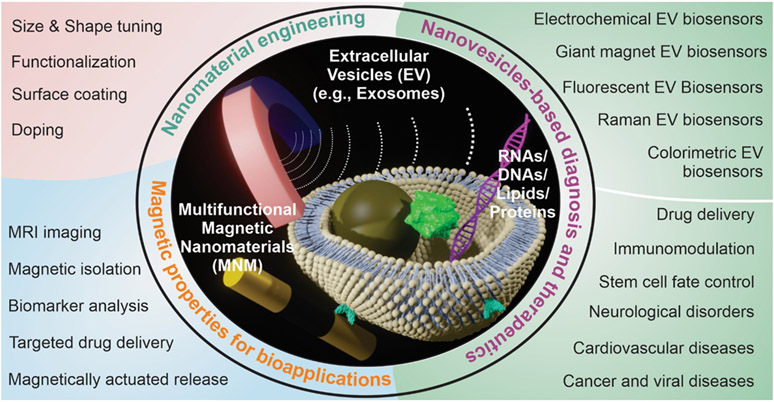
Extracellular vesicles(EVs), which carry various biomolecules such as proteins, lipids, and nucleic acids have rapidly become a promising platform for many biomedical applications. Despite their great potential, the heterogeneity in their surface and size, the high complexity of their cargo biomolecules, and the inefficient uptake by recipient cells remain key barriers to their therapeutic and diagnostic applications. To address these issues, multifunctional nanomaterials, such as magnetic nanomaterials, have tunable physical, chemical, and biological properties that facilitate the next generation of disease diagnosis, drug delivery, tissue engineering, and regenerative medicine based on EVs. Researchers from Rutgers University, The State University of New Jersey, USA, published a review in Small magazine, introducing the latest knowledge about magnetic nanomaterials and their applications in promoting the separation, detection, and delivery of extracellular vesicles and their associated biomolecules. The combination of EVs and magnetic nanomaterials promises to significantly advance biomedical applications and clinical translation.

EVs are critical mediators of intercellular communication and deliver a variety of biomolecules, including nucleic acids, proteins, polysaccharides, and lipids, inspiring numerous therapeutic applications. However, the heterogeneity in size, composition, tissue origin, and cell origin of EVs presents various research opportunities and challenges for their biomedical applications.
In the past two decades, engineered magnetic nanomaterials (MNMs), including 0D nanoparticles, 1D nanorods, 2D nanosheets, and hybrid 3D nanomaterials, have attracted significant interest. They have demonstrated considerable advantages in the separation and targeted delivery of various biomolecules, lipid vesicles, and living organisms. MNMs have been widely used in the separation and purification of biomolecules, biosensors, magnetic mode imaging, and in vitro and in vivo treatments of various diseases, offering clear benefits in overcoming the heterogeneity issues in EV-based applications.
In this review, researchers first briefly introduce the biological background of EVs, the biomolecules and functions of EVs, and the strategies for engineering MNMs for biomedical applications. They then focus on the central question of “how to design MNMs to overcome the heterogeneity of size, composition, and origin of EVs and perform diagnostic and therapeutic applications.” Recent studies have emphasized MNM-based EV capture, separation, purification, concentration, and the use of optical, magnetic, fluorescent, electrical, and electrochemical biosensors for EV-based diagnostic applications. These approaches aim to achieve sensitive, selective, reproducible, and rapid detection of internal biomolecules in EVs. The information obtained from MNM-based biosensors has been used for the multiplexed detection of biomarkers, facilitating precision medicine and the non-invasive monitoring of diseases or biological processes. Most current work focuses on combining MNMs with magnetic fields to provide tissue-specific delivery of EVs for therapeutic applications. The properties of MNMs, such as their response to alternating magnetic fields and heat release, have been leveraged to trigger the release of cytokines within EVs, allowing for more targeted disease treatment. Other advantages of MNMs include iron-stimulated EV secretion, MRI-based EV tracking in vivo, and nano-EL, all of which have enhanced the therapeutic application of EVs in various disease types, including immune, neurological, cardiovascular, and other diseases. However, for each of these directions, there are currently only a few examples of MNM-facilitated EV therapeutic applications, indicating significant potential for further development in therapeutic and diagnostic applications.
Looking ahead, it is critical to apply MNM-based EV biosensors to address important biological questions. For example, early monitoring of neurological diseases is often challenging due to the blood-brain barrier (BBB). Although invasive methods like brain biopsy can identify early neurological diseases, this approach is not preferred by doctors or patients. EVs capable of pass through the BBB may provide a solution for sensitive, noninvasive, and early detection of biomarkers associated with neurological diseases. MNMs can improve sensitivity and selectivity and allow multiplexed detection for higher accuracy. However, no direct studies have yet demonstrated this potential for innovation. Moreover, a robust standard must be established to confirm the performance and reproducibility of MNM-based EV biosensors. Current literature shows inconsistent detection results due to different sample sources and impurities.
The authors also believe that there is substantial potential for MNM-based EV therapeutic applications. Many clinical trials have focused on MNMs and EVs, both of which hold high potential for clinical translation. Despite some advantages, the application of MNM-promoted EV therapeutics remains limited, indicating that this field is still in its early research stages. Given that most diseases require cell-specific and tissue-specific drugs, magnetic field-guided EV delivery is widely applicable to in vivo applications. However, translating this technology to humans requires significant innovation, particularly in developing MF devices for deep tissue penetration. Similar challenges exist for AMF-triggered drug release. For future applications, it may be more practical to start with MNM-promoted, EV-based treatments for diseases and injuries closer to the skin surface and then explore treatments for diseases of visceral organs. Nevertheless, the concept of MNM-promoted EV therapy in an in vivo system will pave the way for the next generation of cell-free therapies as well as precision tissue engineering. Combining diagnostic and therapeutic functions of the MNM-EV system will further provide promising solutions for a variety of applications, including image-guided therapy and other theranostic applications.
Reference:
Yang, Letao et al. “Harnessing the Therapeutic Potential of Extracellular Vesicles for Biomedical Applications Using Multifunctional Magnetic Nanomaterials.” Small (Weinheim an der Bergstrasse, Germany) vol. 18,13 (2022): e2104783. doi:10.1002/smll.202104783
Related Services:
