Extracellular vesicles (EVs) are nanoscale, naturally derived vesicles that contain a variety of bioactive molecules reflecting their origin. These nanovesicles have a natural ability to cross the blood-brain barrier and exhibit excellent biocompatibility, making them prime candidates for brain disease diagnosis, treatment, and drug delivery.
 Recently, researchers published a review titled “Emerging prospects of extracellular vesicles for brain disease theranostics” in the Journal of Controlled Release. The review summarizes strategies and methods for effective drug loading of EVs, focusing on their potential diagnostic and therapeutic applications in brain diseases. It introduces the challenges of current research and further discusses the prospects for clinical translation.
Recently, researchers published a review titled “Emerging prospects of extracellular vesicles for brain disease theranostics” in the Journal of Controlled Release. The review summarizes strategies and methods for effective drug loading of EVs, focusing on their potential diagnostic and therapeutic applications in brain diseases. It introduces the challenges of current research and further discusses the prospects for clinical translation.
Brain diseases including neurodegenerative diseases, stroke, multiple sclerosis, and gliomas remain a serious threat to human health. Despite significant achievements in the development of new diagnostic and therapeutic approaches over the past few decades, effective therapeutic strategies for most brain diseases are still lacking. Drug entry into the brain is a complex process, severely hindered by the blood-brain barrier (BBB), the most strictly regulated interface in the human body. In addition to insufficient brain penetration, systemic toxicity and increased BBB disruption are also major challenges for brain drug delivery technologies.
Remarkable advances in nanotechnology and its applications in biomedicine have led to the development of multifunctional nanotherapeutic systems with the potential to overcome the blood-brain barrier (BBB), enabling high-precision diagnosis and treatment of brain diseases. Various studies focus on developing surface-functionalized biomimetic nanomaterials to penetrate the BBB and target the brain, such as cell membranes, lipoproteins, and extracellular vesicles (EVs). EVs are heterogeneous membrane vesicles that originate from the intracellular lysosomal pathway or are formed by budding from the plasma membrane. They have attracted widespread attention in molecular cell biology and pharmaceutical science. EVs are actively secreted by almost all cells and are present in biological fluids, including blood, saliva, ascites, cerebrospinal fluid, breast milk, and urine. They contain a variety of components, such as nucleic acids, functional proteins, lipids, amino acids, and metabolites, which depend on their biogenesis pathway and cell of origin (Figure 1). Increasing studies have shown that EVs not only serve as messengers for intercellular communication and biomolecule transfer in central nervous system (CNS) physiology, but also participate in the pathological processes of brain diseases. This endogenous communication system represents a promising cell-free strategy for personalized diagnosis and therapy. Furthermore, the unique physicochemical properties of EVs provide multiple advantages for delivering therapeutic agents to the brain. In particular, EVs can efficiently carry a variety of biomolecules across the BBB and penetrate diseased areas in the brain due to their surface proteins and lipid membranes.
Due to the special physiological structure and complexity of the CNS, it is generally challenging for drugs to reach brain tissue for treating brain diseases. Leveraging their unique BBB transport ability, EVs have been widely developed to deliver therapeutic agents to the brain, where they can freely penetrate the blood vessel wall, overcome the BBB, and reach the brain parenchyma. The expression levels of their contents, including proteins, lipids and nucleic acids reflect the physiological, pathological, and functional states of the parent cells, making EVs an ideal non-invasive biomarker for disease progression, early detection of brain diseases, monitoring of disease progression, and personalized treatment guidance.
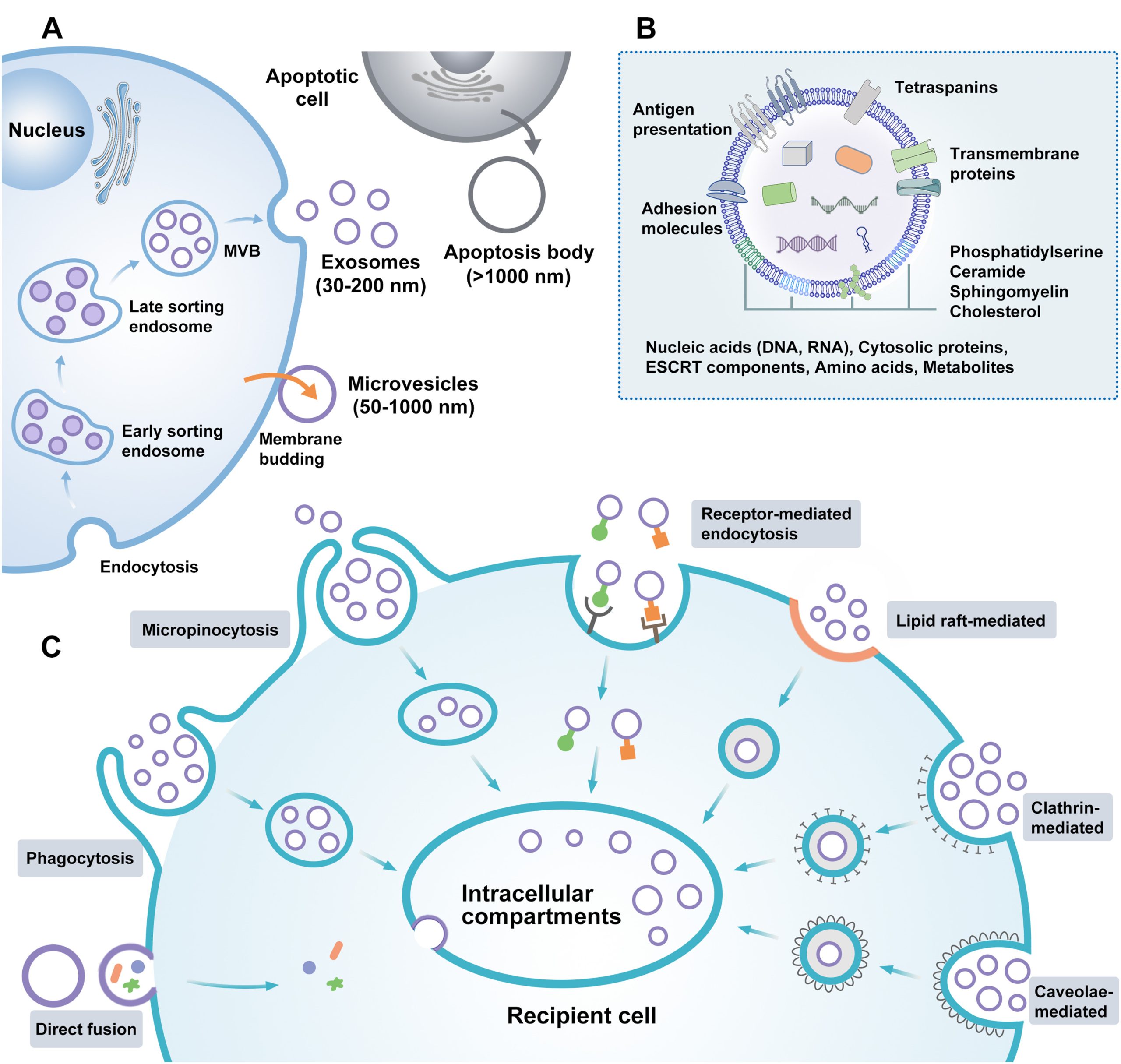 Figure 1 Biogenesis, Composition, and Cellular Uptake Mechanism of EVs
Figure 1 Biogenesis, Composition, and Cellular Uptake Mechanism of EVs
In this review, the researchers introduce the biogenesis and composition of EVs, as well as the main isolation techniques and cellular uptake mechanisms. They discuss the advantages of EVs as delivery vehicles, different cell sources, and modification strategies (Figure 2). The review focuses is on strategies for EVs to overcome the BBB and their applications in the treatment and diagnosis of brain diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, stroke, glioma, and multiple sclerosis. Finally, the current challenges and prospects of EVs for diagnosing and treating brain diseases are briefly summarized and discussed.
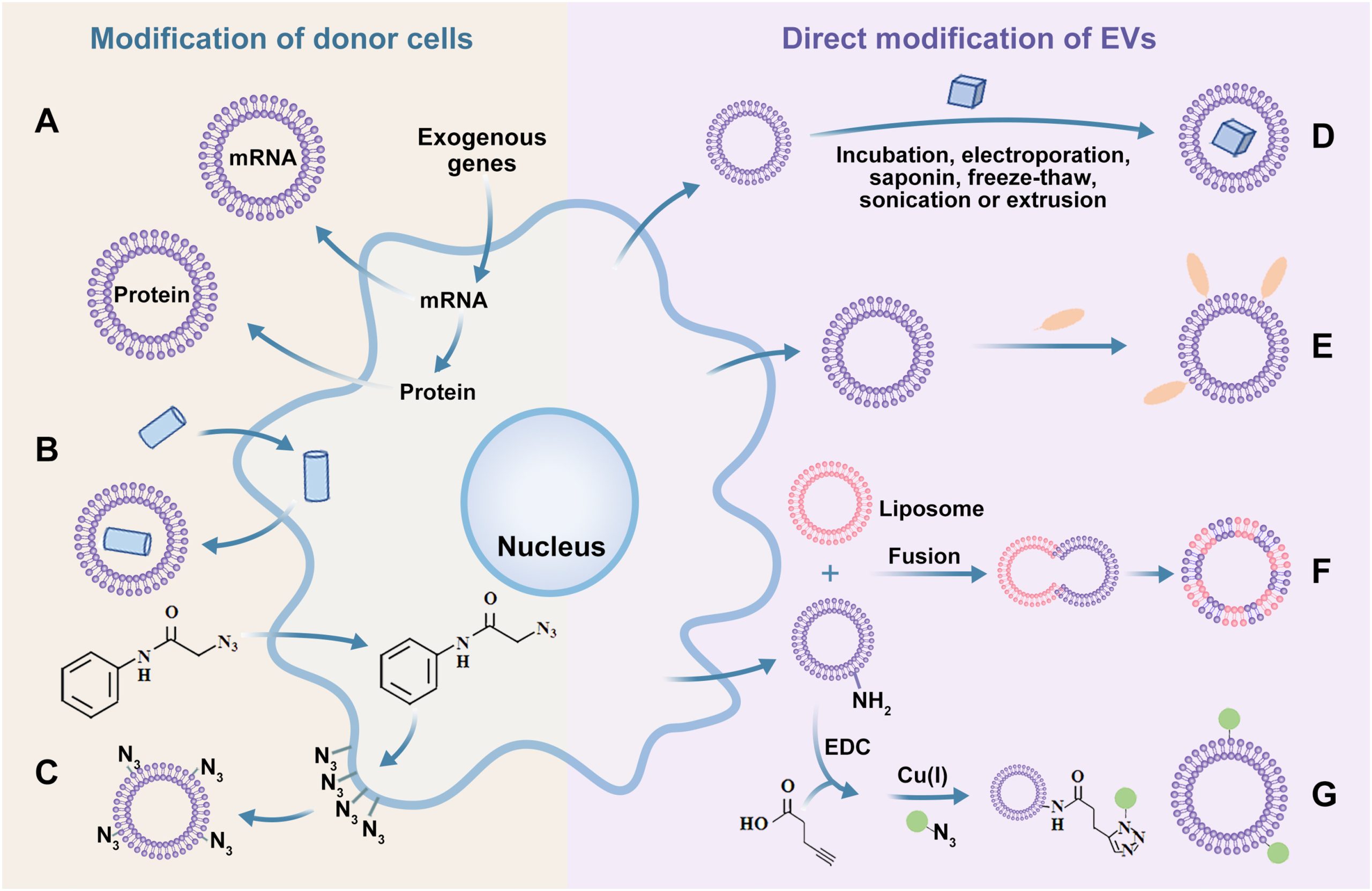 Figure 2 EVs Modification Strategy
Figure 2 EVs Modification Strategy
Reference:
Wang, Ruoning et al. “Emerging prospects of extracellular vesicles for brain disease theranostics.” Journal of controlled release: official journal of the Controlled Release Society vol. 341 (2022): 844-868. doi:10.1016/j.jconrel.2021.12.024
Related Services:
Brain-Targeted Exosome Modification Service
Exosome Carrier-enhanced Crossing of Biological Barriers Advance Summary
