Small extracellular vesicles (sEVs) are nanoscale vesicles. Death receptor 5 (DR5) mediates exogenous apoptosis. Recently, scientists published a paper in Science Advances where they designed single-chain variable fragments (scFvs) that agonize DR5, expressed on the surface of NK cell-derived sEVs. The PDGFR transmembrane domain transports DR5-scFvs to the surface of sEVs. DR5-scFv sEVs can rapidly induce apoptosis in various DR5+ cancer cells, myeloid-derived suppressor cells (MDSCs), and cancer-associated fibroblasts (CAFs). DR5-scFv sEVs specifically migrate to DR5+ tumors both in vitro and in vivo. Systemic delivery of DR5-scFv sEVs significantly inhibited the growth of DR5+ melanoma, liver cancer, and breast cancer, extending the lifespan of mice without apparent toxicity. Compared to DR5 antibodies, DR5-scFv sEVs demonstrated significantly higher efficacy in vivo. In organotypic melanoma, slices from patient-derived samples, DR5-scFv sEVs effectively suppressed melanoma cells and MDSCs while activating CD8+ T cells. This study suggests that DR5-scFv sEVs can inhibit tumor growth by targeting both tumor cells and immune-suppressive stromal cells in the tumor microenvironment (TME).

Chimeric Antigen Receptor (CAR) T cell therapy has made revolutionary progress in treating hematologic malignancies. However, its effectiveness in treating solid tumors remains in question. The major obstacles of CAR-T therapy in solid tumors include limited tumor infiltration of CAR-T cells, the harsh tumor microenvironment (TME), and T cell exhaustion. Additionally, unlike hematologic malignancies, which typically express specific surface markers, solid tumors express tumor-associated antigens at low levels in normal tissues as well. This increases the risk of “targeted but non-tumor-specific” toxicity, as observed in severe toxicities in clinical trials of Her2-CAR T cells and GD2-CAR T cells. Therefore, better cellular therapies are urgently needed for the treatment of solid tumors.
sEVs are relatively stable nanoscale vesicles that can cross biological barriers to reach specific sites. sEVs carry various characteristics of their parent cells and exhibit excellent biocompatibility. sEVs derived from cytotoxic immune cells, such as natural killer (NK) cells, CD8+ T cells, and γδ T cells, can carry perforin, lysosomal enzymes, and other cytotoxic molecules, delivering them to cancer cells. However, unmodified sEVs secreted by immune cells lack tumor-targeting capability. sEVs released by CAR-T cells, which carry CARs on their surface, have shown promising targeting and therapeutic effects against certain cancers in preclinical models.
DR5 is a receptor for Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). DR5 is highly expressed in various cancers, such as liver cancer, melanoma, and pancreatic cancer, while its expression is significantly lower in normal tissues. DR5 is also highly expressed in myeloid-derived suppressor cells (MDSCs), the major immune-suppressive cells in the tumor microenvironment (TME). Activation of DR5 induces apoptosis, making DR5 and its related death receptors attractive targets for cancer therapy. Although cancer patients generally tolerate DR5 agonistic antibodies well, tumor shrinkage is relatively rare among those receiving treatment.
Natural Killer (NK) cells are innate immune cells capable of effectively killing tumor cells. The NK92 cell line, known for its safety profile, has been widely studied and used in clinical trials. NK92 cells belong to the CD56bright NK cell subset and can secrete large amounts of immune-modulatory factors, including interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). sEVs derived from NK92 cells have been reported to carry cytotoxic proteins and exhibit anti-tumor activity. In vivo injection of NK92-derived sEVs can inhibit the growth of B16F10 melanoma in mice.
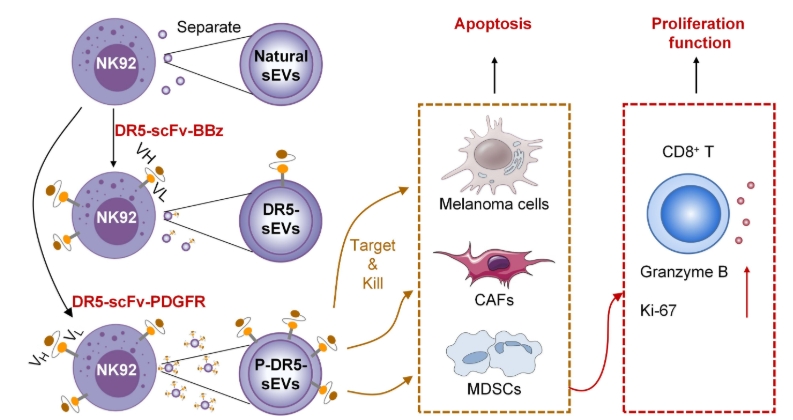 Schematic diagram of DR5-scFv sEVs reprogramming the tumor microenvironment (TME)
Schematic diagram of DR5-scFv sEVs reprogramming the tumor microenvironment (TME)
This study engineered NK92 cells to secrete sEVs that carry DR5-agonistic single-chain variable fragments (scFvs) on their surface. The transmembrane domain of the platelet-derived growth factor receptor (PDGFR) was found to be more efficient than the CD8 transmembrane domain in transporting DR5-scFvs to the surface of sEVs. Live cell imaging studies demonstrated that DR5-scFv sEVs could rapidly induce apoptosis in DR5+ melanoma cells. The engineered sEVs specifically migrated to DR5+ melanoma cells both in vitro and in vivo. Systemic delivery of DR5-scFv sEVs significantly inhibited the growth of multiple cancers and extended the lifespan of treated mice, with no observed adverse effects. Compared to DR5 antibodies, DR5-scFv sEVs exhibited significantly higher cytotoxicity against DR5+ cancer cells both in vitro and in vivo. Additionally, DR5-scFv sEVs significantly suppressed myeloid-derived suppressor cells (MDSCs) and cancer-associated fibroblasts (CAFs) in the tumor microenvironment (TME) and activated CD8+ T cells in patient-derived melanoma slice cultures. Overall, DR5-scFv sEVs inhibited tumor growth by targeting both tumor cells and immune-suppressive stromal cells in the TME.
 DR5-scFvs induce apoptosis in DR5+ tumor cells
DR5-scFvs induce apoptosis in DR5+ tumor cells
sEVs are approximately one millionth the size of T cells, and compared to CAR-T cells, sEVs can more easily penetrate the immune barriers of the tumor microenvironment (TME), reaching immune-privileged sites and solid tumors. This study shows that while DR5 CAR-T cells effectively controlled the growth of A375 melanoma in a nude mouse model through intratumoral injection, systemic delivery showed limited efficacy. In contrast, DR5-scFv sEVs delivered systemically exhibited significant tumor control effects in multiple solid tumor models, with tumor-targeting abilities similar to CAR-T cells. Compared to DR5 antibodies, DR5-scFv sEVs showed higher cytotoxicity, significantly suppressed MDSCs and cancer-associated fibroblasts (CAFs), and activated CD8+ T cells, thereby enhancing the immune response.
DR5-scFv sEVs combine the natural advantages of sEVs with the properties of DR5-scFv, enabling specific targeting of DR5+ tumor cells to induce apoptosis while protecting the scFv from degradation in circulation. By loading more DR5-scFvs onto the surface of sEVs, the tumor-killing effect can be significantly enhanced. Unlike CAR-T cells, sEV delivery does not require lymphatic clearance, resulting in fewer side effects. Additionally, sEVs derived from NK92 cells carry cytotoxic molecules such as perforin and granzymes, as well as signaling molecules like cytokines, chemokines, and miRNAs, which can reprogram the TME and enhance the anti-tumor inflammatory response.
NK92 cells are easy to engineer and suitable for large-scale production, and they have been approved by the U.S. FDA for cancer immunotherapy. This NK92 cell-based sEVs production method not only reduces manufacturing costs but also significantly minimizes batch-to-batch variability. However, sEVs still face challenges in purification and clinical application, including the lack of standardized separation and purification methods, low yields, and product heterogeneity. Furthermore, regulatory requirements for sEVs remain unclear, but there are currently 66 ongoing sEV-related clinical trials, demonstrating their therapeutic potential.
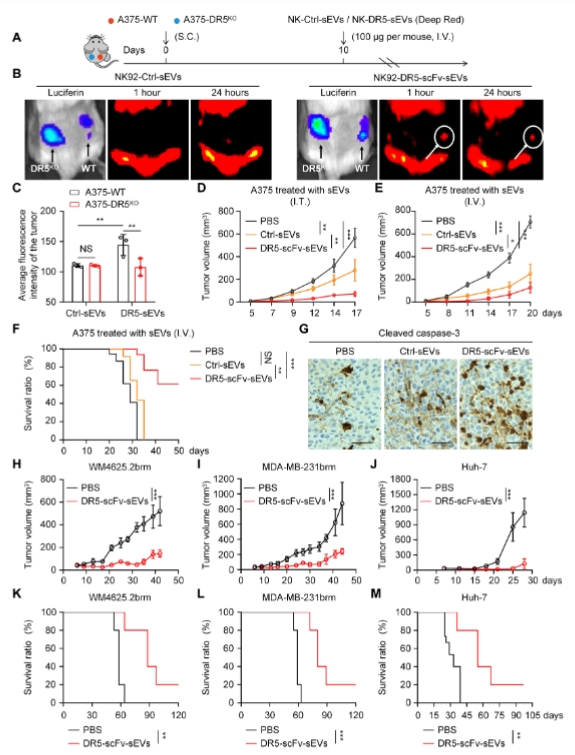 DR5-scFv sEVs migrate to DR5+ melanoma and inhibit tumor growth in vivo
DR5-scFv sEVs migrate to DR5+ melanoma and inhibit tumor growth in vivo
In summary, this study developed an engineered sEV platform that efficiently expresses DR5-scFvs, capable of specifically targeting DR5+ tumor cells, MDSCs, and CAFs, alleviating the immune-suppressive TME, and restoring the normal function of other immune cells. This “ready-to-use” sEVs platform can target multiple cancers by loading different scFvs, providing a universal immunotherapy solution for the treatment of solid tumors.
Reference:
- Guo, Yeye et al. “Engineered extracellular vesicles with DR5 agonistic scFvs simultaneously target tumor and immunosuppressive stromal cells.” Science Advances vol. 11, 3 (2025): eadp9009. doi:10.1126/sciadv.adp9009
- under Open Access license CC BY 4.0, without modification.
Related Services:
