Intercellular communication stands as a pivotal characteristic in tumor progression and metastasis. Extracellular vesicles (EVs), generated by diverse cells including tumor cells, serve as indispensable agents in cell-to-cell communication. They wield influence over tumors by encapsulating and transferring bioactive components, thereby shaping the biological processes and functionalities within both cells and the tumor microenvironment. A comprehensive review by researchers from Professor Raghu Kalluri’s team at the University of Texas MD Anderson Cancer Center delves into the latest advancements concerning EVs. This review explores their role as tumor biomarkers, their functional contributions to tumor progression and metastasis, and the evolving landscape of tumor therapeutics. Published in the top international academic journal Cell on April 13, this review is titled “The Role of Extracellular Vesicles in Cancer”.
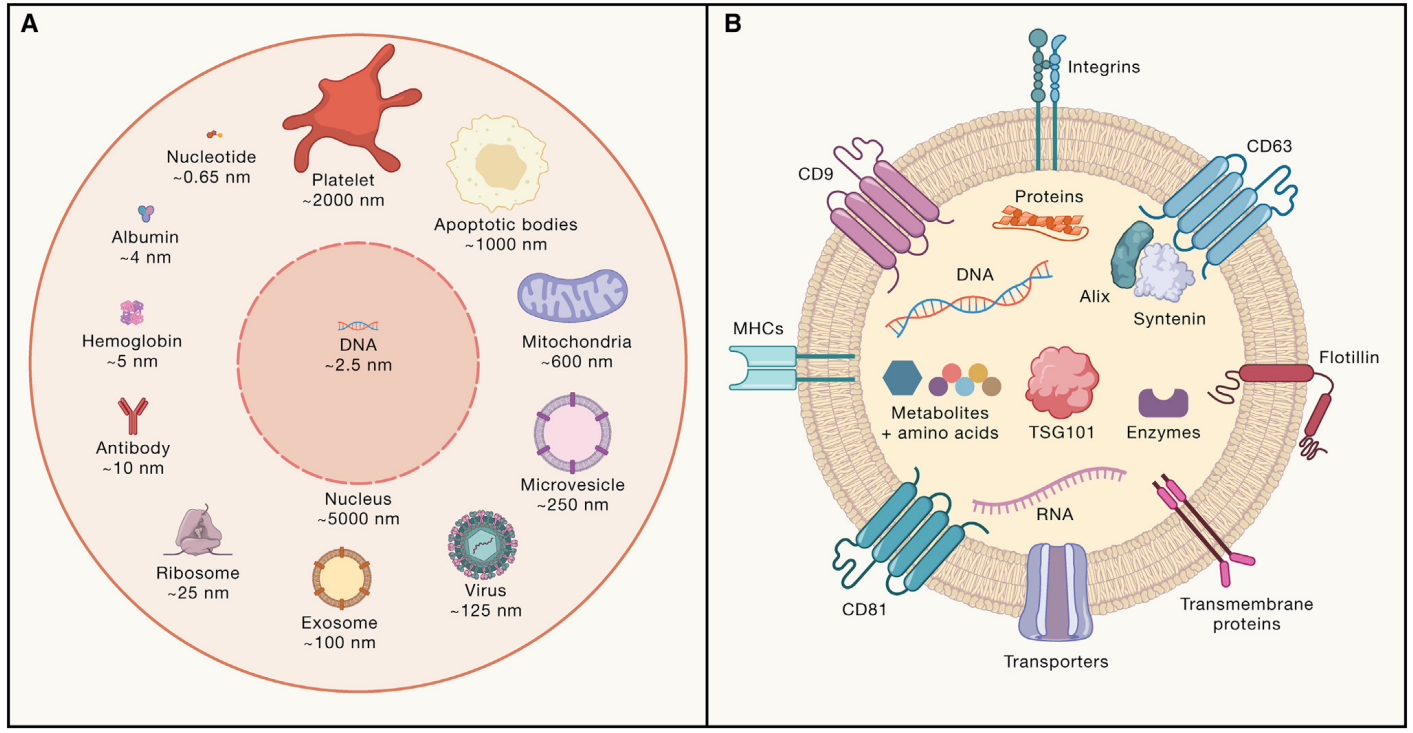 Figure: Classification of Extracellular Vesicles and Cargo Molecules
Figure: Classification of Extracellular Vesicles and Cargo Molecules
The initiation and progression of tumors hinge on communication among precursor cells, malignant tumor cells, various cells within the tumor, and host cells distributed across local tissues and the entire body. This intercellular dialogue has the potential to induce microenvironmental changes, thereby affecting the dissemination of tumor cells. Such signaling mechanisms can occur through the secretion of soluble factors or the exchange of extracellular vesicles (EVs). Initially observed in reticulocytes, EV secretion was believed to be a mechanism for eliminating surplus membrane proteins. However, in-depth research has illuminated the presence of biologically active substances within EVs, encompassing proteins, lipids, metabolites, RNA, and DNA. These substances can be transferred to recipient cells, modulating their functionalities and providing compelling evidence for the pivotal role of EVs as mediators of intercellular communication.
The bidirectional communication facilitated by EVs has been detected among various cell types in both the primary and metastatic tumor microenvironments. EVs exhibit diverse effects across crucial stages of tumor progression, underscoring their heterogeneous origins and compositions. Furthermore, the accumulation of EVs within tumors, their biocompatibility, and the ease of modifying EV cargo have been harnessed to develop innovative EV-based therapeutic strategies. These strategies target multiple facets of the tumor microenvironment.
In this review, the authors succinctly outline the multifaceted roles of EVs in tumor initiation, progression, and metastasis, therapy response, biomarker identification, and the evolution of tumor therapeutics. The authors contend that recent years have witnessed remarkable strides in EV research, unraveling unprecedented insights into EV biology and its intricate involvement in tumor advancement, treatment responsiveness, and metastatic processes. While our comprehension of EV function primarily stems from pathological contexts—i.e., disease states—the enigma of EVs in normal physiological processes and homeostatic tissue functions persists. An additional challenge arises in understanding EV contributions to cancer initiation due to the complexity of precancerous cell types. These cells often resist in vitro expansion and maintenance of their phenotypes, rendering it impossible to isolate and analyze EVs derived from these cells for evaluating their role in cancer inception. Similar challenges are encountered concerning specific cells within the Tumor Microenvironment (TME), including lymphatic endothelial cells, neurons, subsets of immune cells, and Cancer-Associated Fibroblasts (CAFs).
Numerous markers enriched in circulating EVs from early-stage tumor patients and healthy individuals have been identified. Nevertheless, the precise cellular origins of these EVs remain enigmatic. Models capable of in vivo tracking of EVs released by diverse cell populations could significantly contribute to resolving these uncertainties. Additionally, while various mediators of EV biogenesis have been identified in vitro, whether these functions are preserved in vivo or restricted solely to EV secretion remains ambiguous. The identification of mediators governing EV-specific secretion holds the key to dissecting of EV contributions to tumor progression.
Single EV analysis techniques have highlighted the inherent heterogeneity in the size and marker expression of individual EVs. However, it’s noteworthy that most studies delving into the role of EVs in tumors have relied on EVs isolated through rudimentary methods, potentially capturing a heterogeneous mixture of EVs. Notably, EVs exhibit diverse impacts on metastatic growth and biodistribution, contingent upon volumetrically measured surface markers and size, with CD63+ EVs, for instance, harbor a distinct protein composition compared to CD9+ EVs, indicating the possible existence of diverse functional EV subtypes. In the realm of EV technology, rapid strides have been made in recent years. This progress encompasses the meticulous analysis and categorization of individual EVs and the development of methods to separate EVs based on their size and charge. These advancements are poised to facilitate in-depth exploration of EV subtypes and an assessment of their functional roles. Additionally, the emergence of cutting-edge technologies for detecting nucleic acids within individual EVs promises to unravel the intricacies of EV heterogeneity..
To date, EV-based treatments have thus far demonstrated remarkable safety profiles. Unmodified EVs sourced from specific non-malignant cell types do not provoke additional immune responses and can serve as viable allogeneic therapies. Furthermore, the modification of EV cargo, such as expressing CD3 antibodies, enables the engineering of EVs to activate T cells. This innovation suggests that EVs can be tailored to yield readily available allogeneic therapeutics with precise immune targeting or immunomodulatory attributes. The potential of EVs extends even further, with EVs expressing the receptor-binding domain of the SARS-CoV-2 spike protein demonstrating their effectiveness as vaccines. This strategy can be harnessed to utilize tumor antigens to produce EV vaccines. Personalized medicine stands to benefit greatly from this approach, targeting patient-specific mutations as well as more prevalent mutations like KRAS G12D and others.
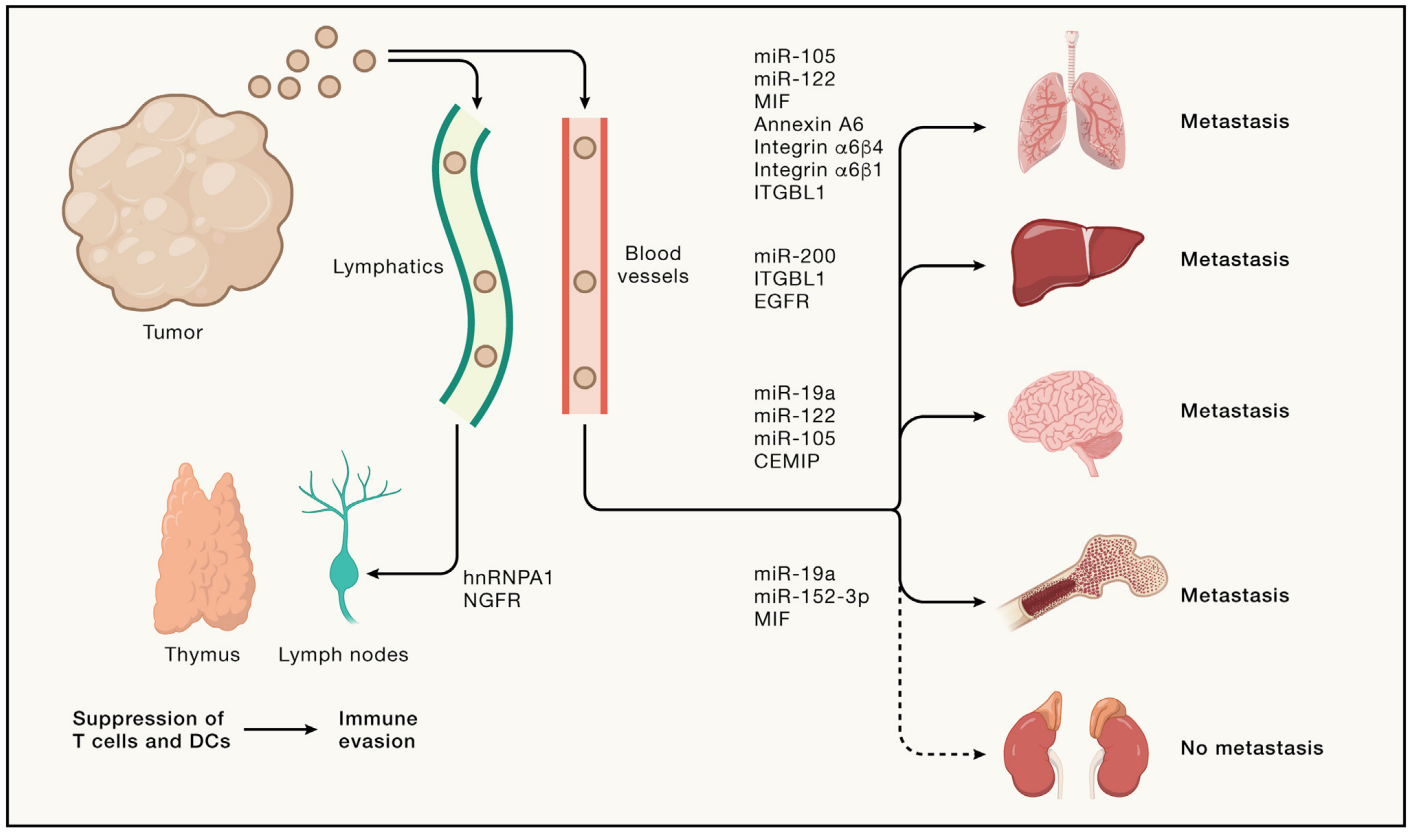 Figure: Extracellular Vesicles in Tumor Metastasis. Tumors release EVs originating from both tumor cells and host cells within the tumor Microenvironment (TME).These EVs embark on a systemic journey, coursing through lymphatic and blood vessels. These EVs interact with lymphoid organs, including the thymus and lymph nodes, affecting T cell activation and Dendritic Cells (DCs), potentially contributing to immune evasion. Additionally, EVs impact metastasis in various organs such as the lungs, liver, brain, and bones, as well as other potential non-metastatic sites, by changing vascular permeability, influencing immune cell recruitment, remodeling the extracellular matrix (ECM), and activating fibroblasts. EVs execute their functions by modifying recipient cells through the transmission of RNA, cytokines, chemokines, growth factors or surface protein signals.
Figure: Extracellular Vesicles in Tumor Metastasis. Tumors release EVs originating from both tumor cells and host cells within the tumor Microenvironment (TME).These EVs embark on a systemic journey, coursing through lymphatic and blood vessels. These EVs interact with lymphoid organs, including the thymus and lymph nodes, affecting T cell activation and Dendritic Cells (DCs), potentially contributing to immune evasion. Additionally, EVs impact metastasis in various organs such as the lungs, liver, brain, and bones, as well as other potential non-metastatic sites, by changing vascular permeability, influencing immune cell recruitment, remodeling the extracellular matrix (ECM), and activating fibroblasts. EVs execute their functions by modifying recipient cells through the transmission of RNA, cytokines, chemokines, growth factors or surface protein signals.
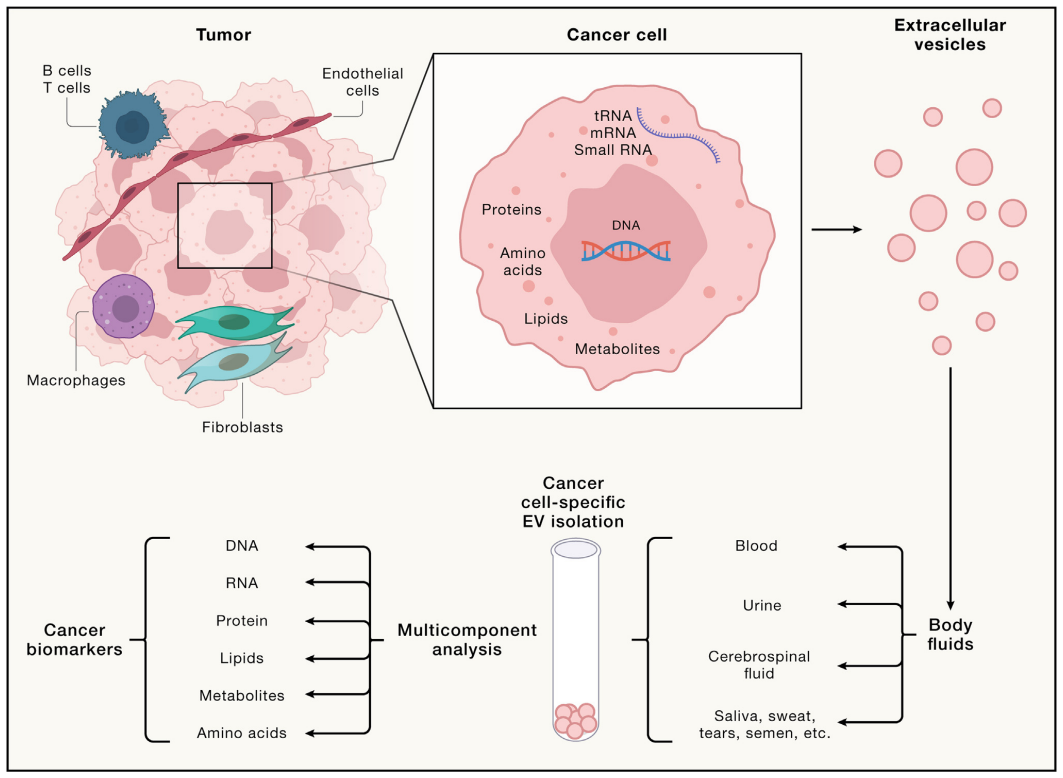 Figure: EVs as Tumor Biomarkers. EVs released by tumor cells carry a distinctive cargo, representing a wide array of tumor cell components, including nucleic acids, proteins, lipids, metabolites, and more. These EVs are present in all body fluids, including blood, urine, cerebrospinal fluid, saliva, sweat, tears, and even semen, and can be enriched using various isolation methods. EVs are conducive to multi-component analysis and reflect the amalgamation of tumor cell by-products, making them invaluable for biomarker research.
Figure: EVs as Tumor Biomarkers. EVs released by tumor cells carry a distinctive cargo, representing a wide array of tumor cell components, including nucleic acids, proteins, lipids, metabolites, and more. These EVs are present in all body fluids, including blood, urine, cerebrospinal fluid, saliva, sweat, tears, and even semen, and can be enriched using various isolation methods. EVs are conducive to multi-component analysis and reflect the amalgamation of tumor cell by-products, making them invaluable for biomarker research.
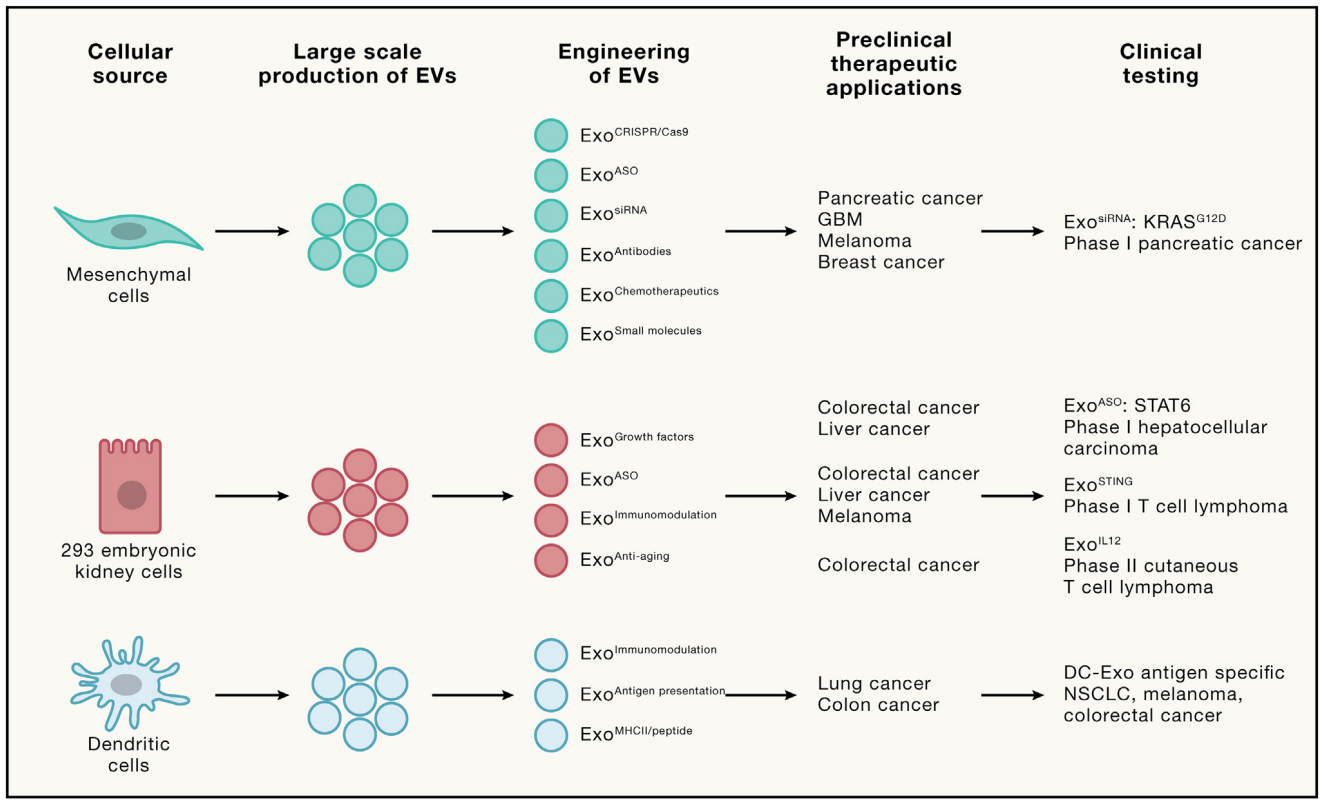 Figure: EV as Anti-tumor Therapeutic Agent. Various cell sources have been utilized for large-scale production of EVs in clinical trials. The engineering of EVs involves adding cargo such as ASO, siRNA, chemotherapy drugs, and more. Furthermore, exosomes are enriched with unique surface protein presentations, including antigens and immune-modifying receptors. Insights gained from preclinical studies in diverse tumor models and types guide the formulation of ongoing clinical trials. EVs present a groundbreaking a new therapeutic platform for tumor treatment, ranging from personalized medicine to immunotherapy and targeted therapy, promising novel levels of safety and efficacy.
Figure: EV as Anti-tumor Therapeutic Agent. Various cell sources have been utilized for large-scale production of EVs in clinical trials. The engineering of EVs involves adding cargo such as ASO, siRNA, chemotherapy drugs, and more. Furthermore, exosomes are enriched with unique surface protein presentations, including antigens and immune-modifying receptors. Insights gained from preclinical studies in diverse tumor models and types guide the formulation of ongoing clinical trials. EVs present a groundbreaking a new therapeutic platform for tumor treatment, ranging from personalized medicine to immunotherapy and targeted therapy, promising novel levels of safety and efficacy.
Reference:
Kalluri R, McAndrews KM. The role of extracellular vesicles in cancer. Cell. 2023;186(8):1610-1626. doi:10.1016/j.cell.2023.03.010
Related Services:
