On September 2, 2024, Nature Materials published a study titled “Tumour-derived small extracellular vesicles act as a barrier to therapeutic nanoparticle delivery.”

This research challenges traditional views by revealing that tumor-secreted exosomes serve as a biological barrier, playing a key role in cancer resistance to therapy. These exosomes bind to therapeutic drugs, diverting them away from tumors and toward degradation in Kupffer cells in the liver, thereby reducing drug accumulation in tumors and diminishing therapeutic effectiveness.
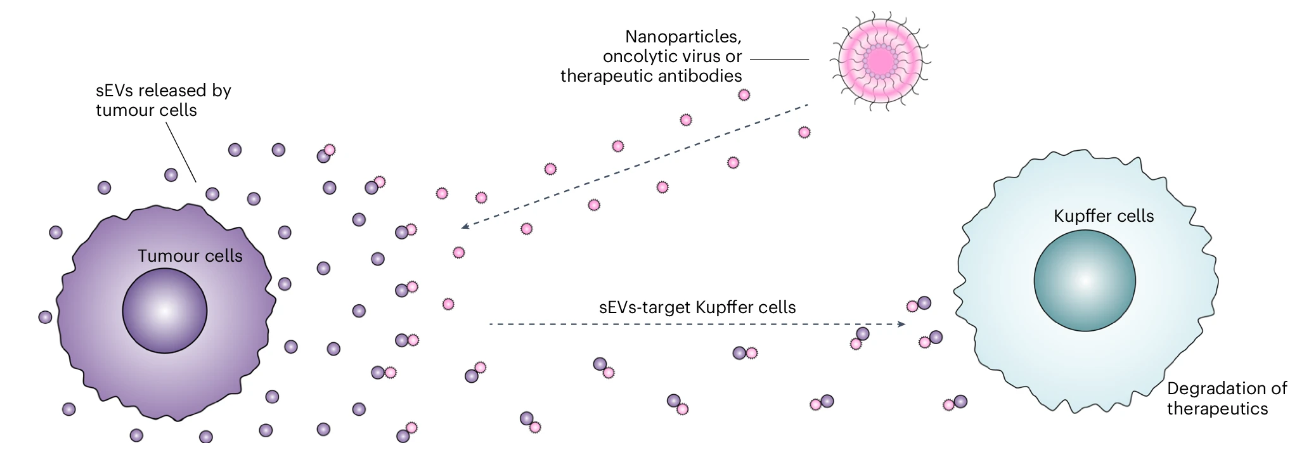 Figure: Illustration of the defense mechanism mediated by tumor-secreted small extracellular vesicles (sEVs).
Figure: Illustration of the defense mechanism mediated by tumor-secreted small extracellular vesicles (sEVs).
Nanoparticle drug delivery systems have been widely applied in clinical settings, but a core challenge is how to effectively deliver a sufficient amount of nanoparticles to tumor sites to achieve optimal therapeutic effects. Although various strategies have been developed—including optimizing the size, shape, and surface chemistry of nanoparticles, as well as enhancing their accumulation in tumors through the enhanced permeability and retention (EPR) effect or ligand-targeting mechanisms— research has shown that only a very small fraction of intravenously injected nanoparticles ultimately reach and accumulate in solid tumors. This inefficiency significantly limits the potential of nanoparticles in tumor treatment.
In recent years, researchers have begun to focus on certain characteristics of the tumor microenvironment, such as the dense extracellular matrix, solid stress, and abnormal vascular structures, which significantly impact nanoparticle accumulation in tumors. Despite tremendous efforts to overcome these obstacles, progress remains limited, primarily due to the many unknowns surrounding the mechanisms of drug delivery to tumor tissue. Small extracellular vesicles (sEVs) are a class of small vesicles secreted by cells and found in various tissue environments, including tumors. Studies have shown that tumor cells are one of the primary sources of sEVs, which are typically present at higher concentrations in the tumor microenvironment than in healthy tissue. However, the physicochemical significance of the high concentration of exosomes at tumor sites has long been overlooked.
Through a series of experiments, the paper reveals how tumor-derived sEVs hinder nanoparticle accumulation in tumor tissues. First, the researchers validated this hypothesis in a mouse model. By knocking out the Rab27a gene using CRISPR-Cas9 technology, the study demonstrated that Rab27a knockout significantly reduced sEV secretion from tumor cells in mice, directly leading to a substantial increase in nanoparticle accumulation in the tumor. Moreover, the experiments found that Rab27a gene knockout not only reduced sEV accumulation in liver Kupffer cells but also increased nanoparticle uptake by tumor cells and tumor-infiltrating immune cells.
A key finding of the study is that sEVs can bind to nanoparticles and transport them to Kupffer cells in the liver for degradation through physical forces such as van der Waals forces, thereby reducing nanoparticle accumulation in tumors. The researchers observed that the binding of sEVs to nanoparticles increased the size of the complexes, which may further affect their permeability and accumulation in tumors. However, experimental results indicated that the inhibition of nanoparticle uptake by sEVs was not solely due to the size increase; it also involved molecular-level interactions. For example, by using antibodies to block the adhesion molecule ICAM-1 on the surface of sEVs, the researchers further confirmed the role of ICAM-1 in the interaction between sEVs and nanoparticles and their transport to Kupffer cells. This molecular mechanism highlights the active role of tumors in resisting treatment strategies.
Next, the researchers explored the possibility that inhibiting the secretion of sEVs could reduce the levels of sEVs that bind to nanoparticles, thereby significantly enhancing nanoparticle delivery efficiency and improving their therapeutic effectiveness in tumor treatments. Notably, they proposed combining Rab27a knockout with the co-delivery of therapeutic mRNA, such as Pten or Sting mRNA, which showed significant antitumor effects in mouse models without causing notable organ toxicity. The researchers further examined the applicability of the sEV barrier effect in other therapeutic methods. The results indicated that sEVs not only hindered the delivery of organic nanoparticles (such as liposomes and PLGA nanoparticles) but also had significant effects on inorganic nanoparticles (such as nanoparticles and silica nanoparticles) and other tumor therapies, including oncolytic virus therapy and antibody therapy. This discovery broadens the scope of sEVs as therapeutic barriers, suggesting that targeted treatment against sEVs could be an effective strategy for enhancing the efficacy of various tumor therapies.
Overall, this paper reveals how tumor-derived sEVs hinder nanoparticle delivery through detailed experiments and in-depth mechanistic analysis. It proposes potential strategies to improve the therapeutic outcomes of nanoparticles in tumor treatments by disrupting this barrier. This research not only enhances the understanding of the role of sEVs in the tumor microenvironment but also offers new insights for future nanodrug development. The study holds significant implications for various tumor therapies, and future research may further explore effective methods to overcome the sEV barrier, investigate the role of sEVs in other types of tumors, and examine their interactions with different treatment methods, thereby advancing the success of tumor therapies.
Reference:
Gong, Ningqiang et al. “Tumour-derived small extracellular vesicles act as a barrier to therapeutic nanoparticle delivery.” Nature materials, 10.1038/s41563-024-01961-6. 2 Sep. 2024, doi:10.1038/s41563-024-01961-6
Related Services:
Nanoparticle Tracking Analysis-based Exosome Characterization
