Gene therapy is a therapy that treats genetic diseases by introducing genetic material (such as the expression system of therapeutic proteins or editing tools to correct wrong genes in diseased cells). The development of this field has brought hopeful treatment for previously incurable diseases, such as genetic diseases, cancer, and autoimmune diseases. So far, at least 943 gene therapy clinical trials have been conducted around the world.
Delivery is essential for gene therapy. The cell membrane formsan impermeable barrier to negatively charged nucleic acids. Moreover, the genetic material is very fragile in the human body, so it must be protected to be effective. mRNA and viral or non-viral plasmid DNA are usually the genetic materials used in gene therapy. For example, adeno-associated virus (AAV) vectors are widely used. However, AAV vectors induce host immune responses, leading to rapid degradation or neutralization of the vector, and severely impairing the efficiency of its gene therapy. Encapsulation of nanostructures (such as exosomes) can protect AAV vectors from the host’s immune system and deliver them into cells through the plasma membrane. Professor Xia Jiang from The Chinese University of Hong Kong published a review in Nanoscale, describing the process of exosomes as carriers for encapsulation and delivery of gene vectors to cells, as well as related disease treatment methods, the pros and cons of exosomes as carriers of various genetic materials, and the strategy of engineering exosomes to increase load and delivery , The method of maximizing the yield of gene-loaded exosomes, and the discussion of the future prospects of exosome-mediated targeted gene therapy (Figure 1).
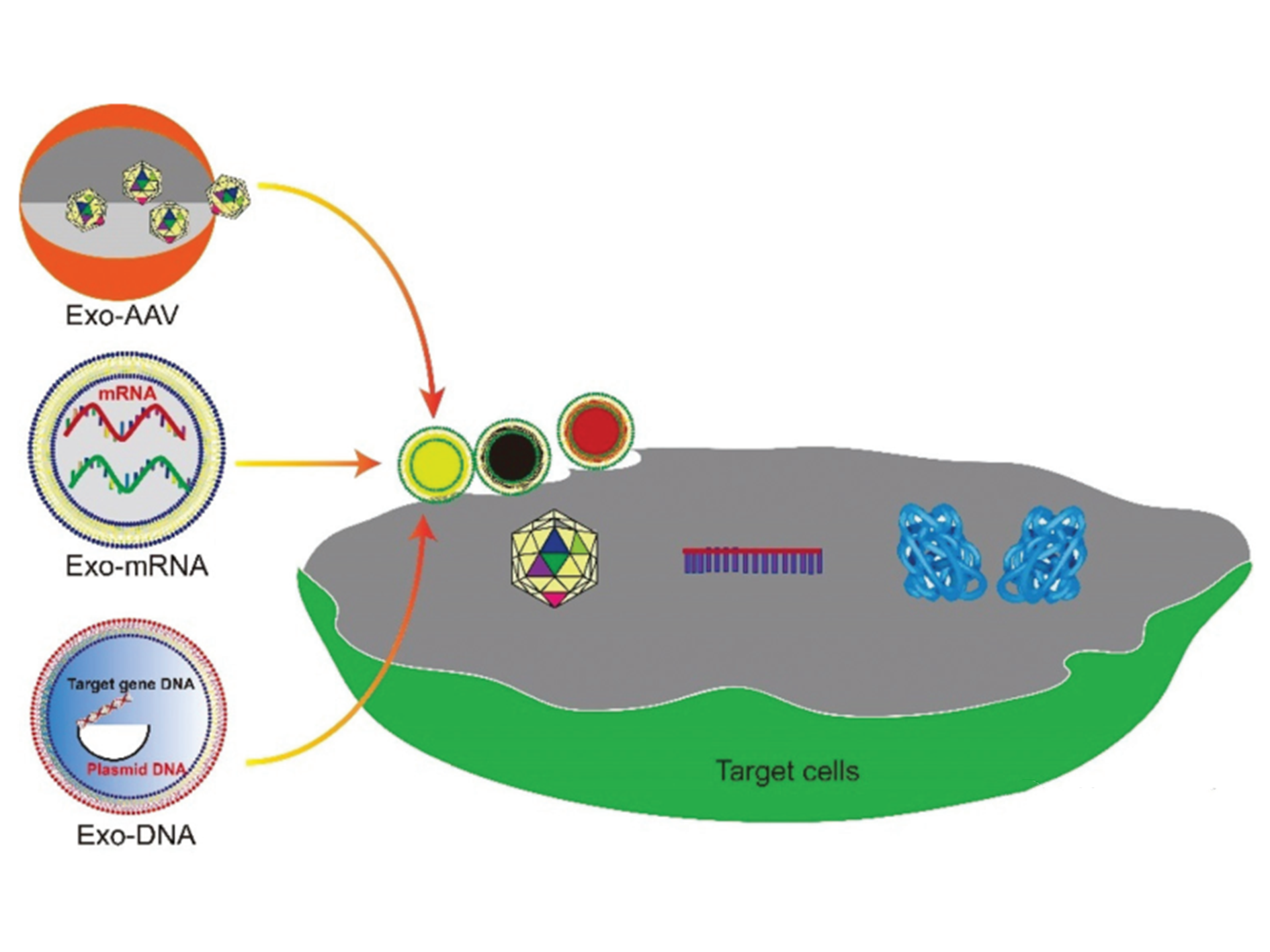
Figure 1. Schematic diagram of exosome-mediated gene delivery strategy
1. Exosome-related AAV vectors are used for gene therapy
According to the source, gene delivery vectors can be divided into viral systems or non-viral systems (Figure 2). Virus-mediated gene delivery systems use replication-deficient modified viruses, but can deliver DNA expression, including adenovirus, retrovirus, and lentivirus. Traditional non-viral vectors include micelles, liposomes, dendrimers, and carbon nanotubes. Recently, extracellular vesicles secreted by cells, especially exosomes, have been used as non-viral vectors for gene delivery, and have shown high biocompatibility, low clearance rate, and the desired capability of cell-targeted delivery. Hybrid gene delivery technologies, such as exosome-AAV hybridization, combine viral vectors with non-viral vectors and show significantly reduced immunogenicity. This article focuses on gene delivery vectors based on viral vesicles and exosomes.
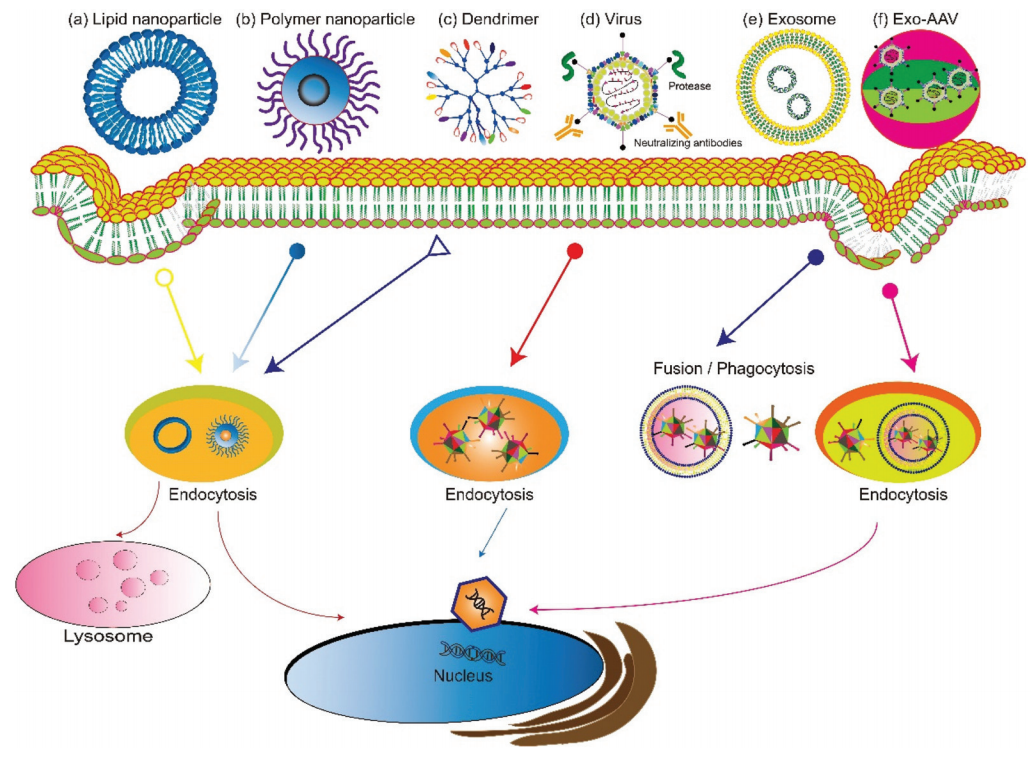
Figure 2. Overview of gene delivery vectors
1.1 Exosomes for gene delivery
Exosomes are vesicles wrapped in a lipid bilayer with a diameter of 40-160 nm, which circulate in the extracellular environment. They originate from the internal budding of the late endosomal membrane and are released by all cell types. Therefore, exosomes are natural carriers for cell-to-cell communication. Then, this feature entices researchers to develop exosome-based drug delivery systems. Compared with other gene delivery systems, exosomes are advantageous for the following reasons. First, exosomes are multifunctional carriers that can encapsulate and deliver various biological cargo, such as small RNA, mRNA, and protein. Second, exosomes have the natural ability to cross biological barriers (e.g. the blood-brain barrier). They can also migrate to tissues or areas where no blood is supplied, such as a dense cartilage matrix. In addition, after reaching the target tissue, exosomes can stay there for a long time. The low clearance rate is attributed to the biocompatibility of exosomes. Another important feature is that exosomes can be genetically engineered. Various purposes can be achieved by modifying the surface proteins of exosomes. For example, peptides that target cells or tissues can be attached to the surface of exosomes to achieve selective targeting to specific tissues and avoid unnecessary accumulation in other organs, thereby reducing systemic toxicity. Studies have shown that targeted gene therapy based on exosomes can enhance therapeutic efficacy and safety. For instance, the researchers have designed an exosome-based chondrocyte-targeted miRNA delivery system for cartilage defect repair. In this work, chondrocyte affinity peptide (CAP) is expressed on the surface of exosomes, and miR-140 is loaded inside exosomes. Compared with the miRNA encapsulated in exosomes without surface modification, the intra-articular injection of CAP-exosomes/miRNA complexes showed selectivity of cartilage and can be long-term enriched there. The therapeutic efficacy of cartilage defects was significantly improved.
1.2 Exosome-related AAV for gene therapy
The combination with exosomes can reduce toxicity and the rapid clearance of AAV vectors. AAV vectors have disadvantages such as rapid clearance of anti-AAV antibodies in the body and off-target delivery (usually to the liver). Maguire et al. proved in 2012 that part of the AAV vector is related to microvesicles/exosomes. Under the electron microscope, the AAV capsid is associated with the surface and interior of the microvesicles. The exosome-related AAV (Exo-AAV) purified from the cell culture medium (Figure 3) is superior to conventional purified AAV vectors in terms of transduction efficiency. A significant problem of AAV vectors is that they induce potent long-term humoral responses, including neutralizing antibodies (NAbs) that bind to AAV vectors and inhibit transduction, and non-Nabs that label viruses. Therefore, NAb will adversely affect the efficacy of AAV-mediated gene therapy and the distribution and safety of AAV vectors. In contrast to traditional AAV, exo-AAV is more resistant to neutralizing anti-AAV antibodies, which has been proved by experiments. Both in vitro and in vivo, exosome-related AAV vectors are more resistant to NAb than AAV. Be it in the presence and absence of Nabs, AAV9Exo-SERCA2a is superior to conventional AAV vectors in terms of the resident heart function. The delivery of Exo-AAV8 gene reduces the therapeutic dose and achieves effective transduction. Exo-AAV also shows higher transduction efficiency in the central nervous system, the auditory system, and the vestibular system.
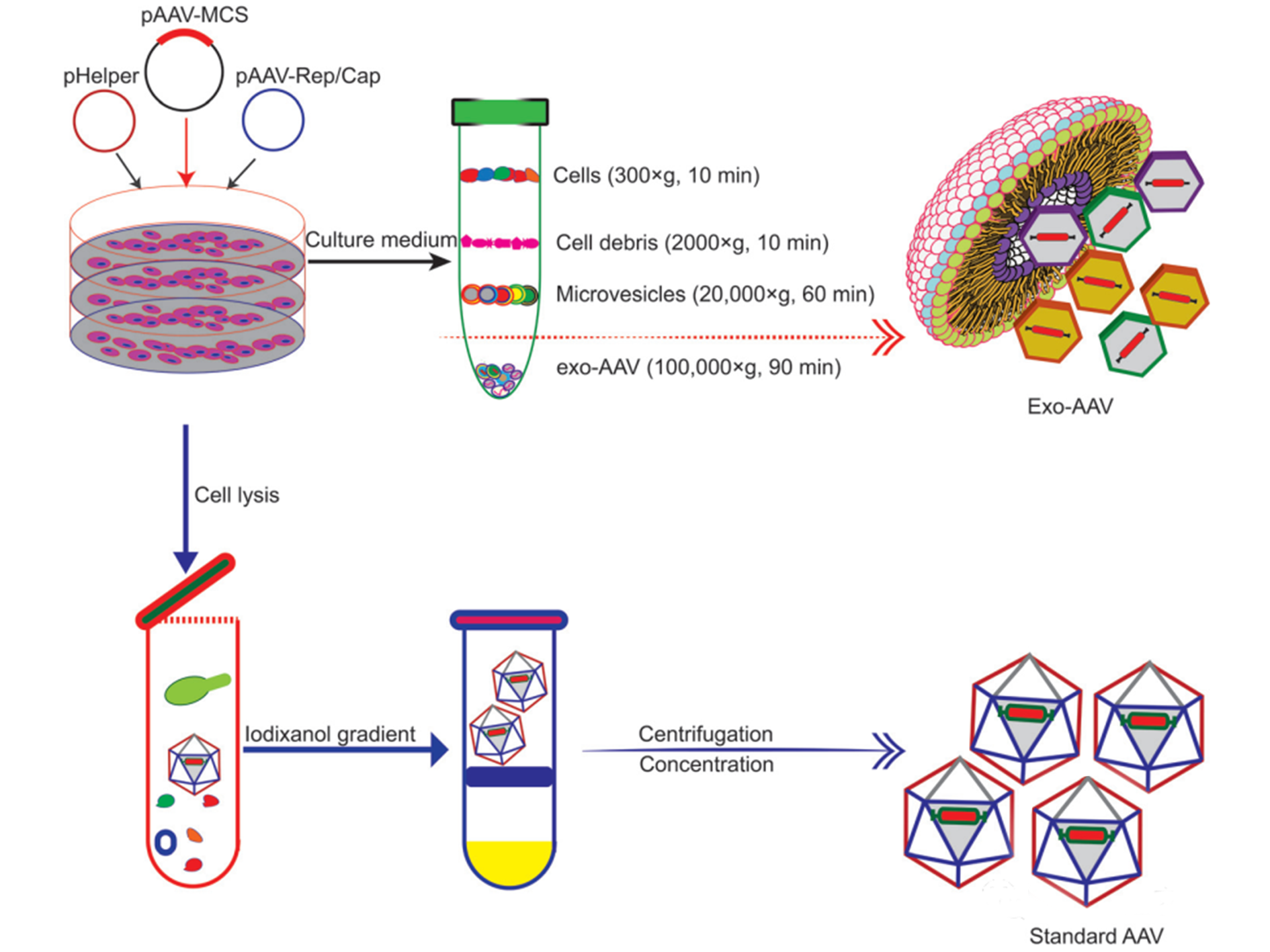
Figure 3. Schematic diagrams showing different procedures for producing and purifying Exo-AAV (top) and AAV alone (bottom) respectively
2. Exosome-mediated CRISPR-Cas9 delivery
Under the guidance of the CRISPR sequence, the Cas9 enzyme can edit genomic DNA in cells with unprecedented precision, efficiency, and flexibility. However, the CRSPR-Cas9 system designed in vitro needs to be delivered to target cells and organisms for gene therapy. The delivery of the CRISPR-Cas9 system can be achieved through physical interactions, chemical modifications, and biological vectors. The CRISPR-Cas9 system can be transported into cells through a short gap in the lipid bilayer which is caused by physical methods, such as electroporation, hydrodynamic injection, and membrane deformation. Although simple and easy, it may cause irreversible physiological damages to the cell membrane. The chemical modification of CAS9-sgRNA will also enable it to be delivered to cells, and biological vectors such as bacteriophages, viruses, and extracellular vesicles also can mediate the delivery of the CRISPR-Cas system to cells.
2.1 Exosomes as CRISPR-Cas9 plasmid vectors
Compared with viral vectors, exosomes from certain cell sources can selectively deliver cargo to tumor tissues. Kim et al. reported that exosomes derived from cancer cells can transport the CRISPR-Cas9 system to target tumors.
2.2 Engineered exosomes for CRISPR-Cas9 delivery
Natural exosomes are not designed to deliver large molecules, such as plasmids. Therefore, the plasmid load in exosomes is low. As a result, exosomes need to be engineered to increase the efficiency and capacity of the plasmid. Engineered exosomes also endow these transport nanoparticles with targeting capabilities that can transport cargo to specific cells or tissues. The marker proteins CD63 and CD9 expressed on the surface of exosomes are usually selected as target proteins for exosome engineering.
2.3 Exosomes-liposome hybrids for gene delivery
The nanoscale size of exosomes (40–160 nm) makes them a qualified carrier for small therapeutic agents such as compounds, siRNA, and miRNA. For large molecules, such as CRISPR-Cas9 expression plasmids with a minimum size of 5-6 kb, the capacity of natural exosomes is still relatively low. On the other hand, liposomes can encapsulate and deliver large plasmids, but due to the non-natural nature of lipids, they have high cytotoxicity. The fusion of the lipid bilayer of the exosomal membrane and liposomes forms an exosome-liposome hybrid, which can encapsulate and deliver large DNA molecules, such as CRISPR-Cas9 expression plasmids, and also reduce the liposome Toxicity issues (Figure 4). Therefore, the hybridization of exosomes-liposomes expands the application range of exosomes in drug delivery.
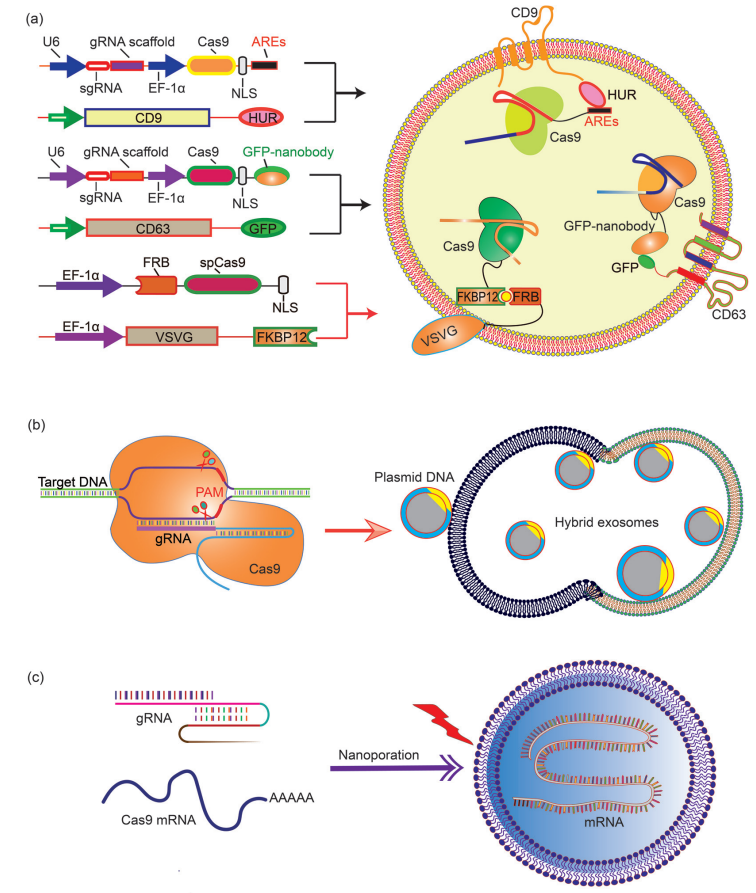
Figure 4. Encapsulation of large nucleic acids in exosomes or exosomes-derived vesicles
3. Exosome-mediated mRNA delivery for gene editing
Encapsulating large mRNA transcripts into exosomes is a difficult task. Although naturally secreted exosomes contain a variety of RNAs, including miRNA, ncRNA, and fragments, most of the fragments are small. Large mRNA molecules can be transferred from red blood cells to exosomes by electroporation, but this process is still inefficient. In view of this obstacle, Yang et al. proposed a method for large-scale production of exosomes carrying functional mRNA. This work developed a cell nanoperforated biochip with nanochannels with a diameter of about 500 nm. The author transfected cells with plasmid DNA and stimulated the transfected cells with local and transient electrical stimulation. This nanoporation procedure promotes the release of exosomes carrying transcribed mRNA and targeting peptides from the cell, resulting in up to a 50-fold increase in exosomes and an increase in exosomal mRNA transcripts by more than 103-fold, and each exosome is estimated to deliver 2-10 complete functional mRNAs.
Conclusions and opinions
Tissue or cell-specific delivery of gene vectors by the designed carriers can realize an advanced version of gene therapy with reduced toxicity or risk. Vectors derived from cell sources and exosomes can mediate the delivery of various gene therapy molecules or vectors. In particular, through genetic or chemical engineering of exosomes, specific recognition sequences can be installed on the outside of exosomes to guide the targeted delivery of goods, or exosomal marker proteins can be used to design protein-protein interactions to enrich target proteins. In addition, engineered exosomes with nucleic acid binding properties will also help the encapsulation of plasmids or vectors and improve loading efficiency. Fusion of exosomes and liposomes will form vesicles that can encapsulate large plasmids or mRNA transcripts while retaining the advantages of exosomes, such as biocompatibility and targeting ability. As an important step towards clinical trials, progress has also been made in achieving large-scale production of exosomes or drug-loaded exosomes. Breakthroughs in instruments and procedures such as nano-perforation have also solved the bottleneck in loading and production. In summary, with the development and optimization of gene editing tools, continuous development of exosome-based vector systems will provide the impetus for targeted gene therapy, so that it can be used as a viable treatment in vitro and in vivo for future refractory diseases (Figure 5).
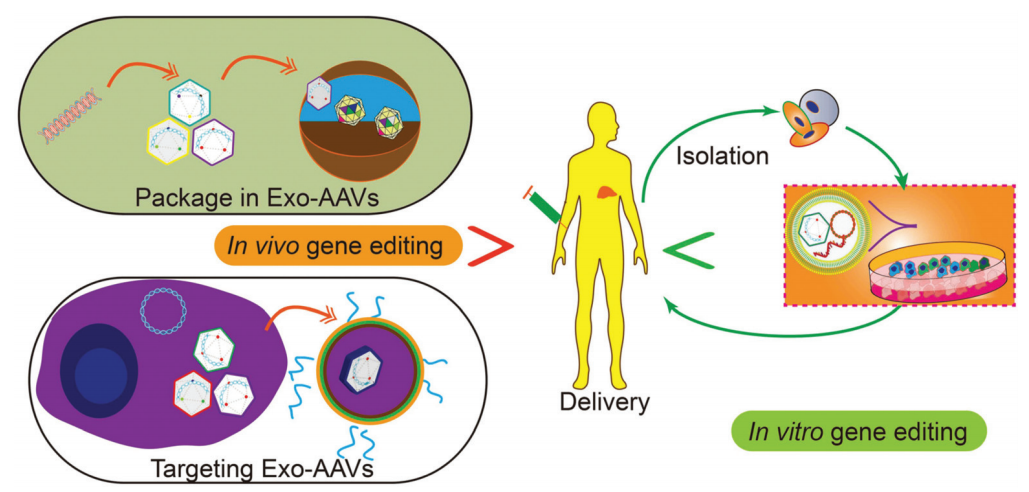
Figure 5. Potential applications of gene therapy in future
