Poor tumor invasion, progression of exhaustion, and antigen insufficiency are common mechanisms that limit the efficacy of chimeric antigen receptor (CAR)-T cells. Delivery of pattern recognition receptor agonists is a strategy to improve immune function. However, targeting these agonists to immune cells is challenging, and off-target signals in cancer cells can be harmful. Recently, Professor Andy J. Minn of the University of Pennsylvania and Carl H June, an expert in CAR-T, co-published an article in the Cell magazine. It reported that CAR-T cells, which express immunostimulatory RNA RN7SL1, can achieve enhanced efficacy in a solid tumor mouse model with loss of CAR antigen by improving the function of autonomous CAR-T cells and promoting endogenous immunity through preferential uptake of extracellular vesicles containing RN7SL1 with innate immune cells (rather than tumor cells).

Chimeric antigen receptor (CAR)-T cells have made remarkable achievements in treating a small number of hematopoietic cancers, but they are still ineffective against most solid tumors. Factors leading to poor efficacy of treating solid tumors include the limited expansion and persistence of CAR-T cells in the tumor microenvironment (TME), which has prompted researchers to improve the intrinsic function of CAR-T cells. However, even when CAR-T cells do successfully bind to tumor targets, the growth of cancer cells that have lost CAR antigen expression can lead to drug resistance. In contrast to adoptive cell therapy, immune checkpoint blockade (ICB) usually stimulates endogenous T cells with multiple specificities to produce a polyclonal anti-tumor response. For CAR-T cell and ICB therapy, the expression of negative immunomodulatory proteins (such as PDL1) and the production of suppressive cytokines (such as TGFB) in TME are additional obstacles that hinder the response to immunotherapy. Therefore, the strategy that simultaneously uses CAR-T cells, enhances endogenous T cell function, and counteracts common inhibitory mechanisms may provide an effective combination method to improve the immunotherapy response to solid tumors.
Damage-related molecular patterns (DAMP) act as ligands for pattern recognition receptors (PRR), which signal tissue damage and/or pathogen invasion. The activation of PRR can initiate the innate immune response, which is a prerequisite for successfully developing adaptive immunity. RIG-I and MDA5 are cytoplasmic PRRs, which usually recognize virus-encoded double-stranded RNA (dsRNA) through 5′-triphosphate. After activation, these PRRs aggregate with the MAVS signaling platform to enhance the production of interferon (IFN) and induce the transcription of IFN-stimulating genes (ISG). This innate immune signal is particularly important for the effective activation of myeloid cells and dendritic cells (DC) and T cells. Recent evidence suggests that endogenous nucleic acids can also act as DAMP to activate PRR. RN7SL1 (7SL) is a highly structured non-coding RNA that exists in all cell types and is conserved from humans to bacteria. Under steady-state conditions, RN7SL1 acts as a scaffold essential for protein translation, in which several types of RNA-binding proteins protect RN7SL1 from the recognition of cytoplasmic RNA PRR. However, under pathological conditions, the reduced interaction with RNA-binding proteins makes RN7SL1 unprotected and secreted by extracellular vesicles (EV) such as exosomes. Therefore, the unshielded RN7SL1 mimics viral RNA to activate the RIG-I-dependent inflammatory response in cancer cells and bone marrow cells.
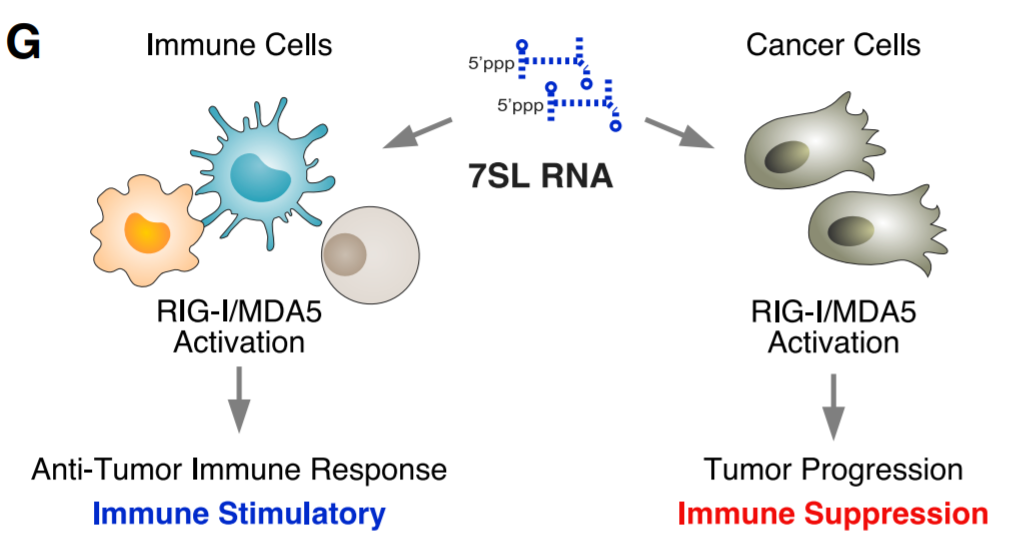
Model for how PRR signaling affects different cellular compartments within the TME
Although DAMP can activate PRR, their effectiveness in enhancing anti-tumor immunity depends on many factors. First, the consequences of inducing type I interferon (IFN-I) or type II interferon response in immune cells and cancer cells could be complex, or even the opposite. Although the PRR and IFN signals in immune cells (such as DC) can have immunostimulatory effects, the signals in cancer cells can drive cancer progression and immunotherapy resistance. The immunosuppressive effect that cancer cells internally activate ISG is believed to occur through a variety of mechanisms, including induction of immunosuppressive genes and genes that affect immune-mediated tumor killing. Secondly, due to the low mutation load and immune editing in most human cancers, the lack of powerful neoantigens will make the endogenous T cell pool incapable of effectively eradicating tumors. This lack of strong neoantigens severely limits the utility of using PRR activation to enhance endogenous T cell priming. Therefore, it is necessary to direct PRR signals to immune cells rather than cancer cells, and to develop methods to deliver new antigens to tumors to maximize the efficacy of cancer immunotherapy.
The study showed how to resolve the complexity of IFN/PRR signals and then modify CAR-T cells to deploy RN7SL1 to re-encode these signals in TME, thereby removing multiple limitations of CAR-T cells against solid tumors. RN7SL1 CAR-T cells exhibit enhanced intrinsic functions, selectively activate RIG-I in immune cells, and can be further equipped to co-deliver peptide antigens. As a result, a multi-armored CAR-T cell is produced, which can effectively infiltrate tumors, activate bone marrow cells and DCs, provide antigens for TME, and activate endogenous T cells to inhibit solid tumors under the threat of CAR antigen loss.
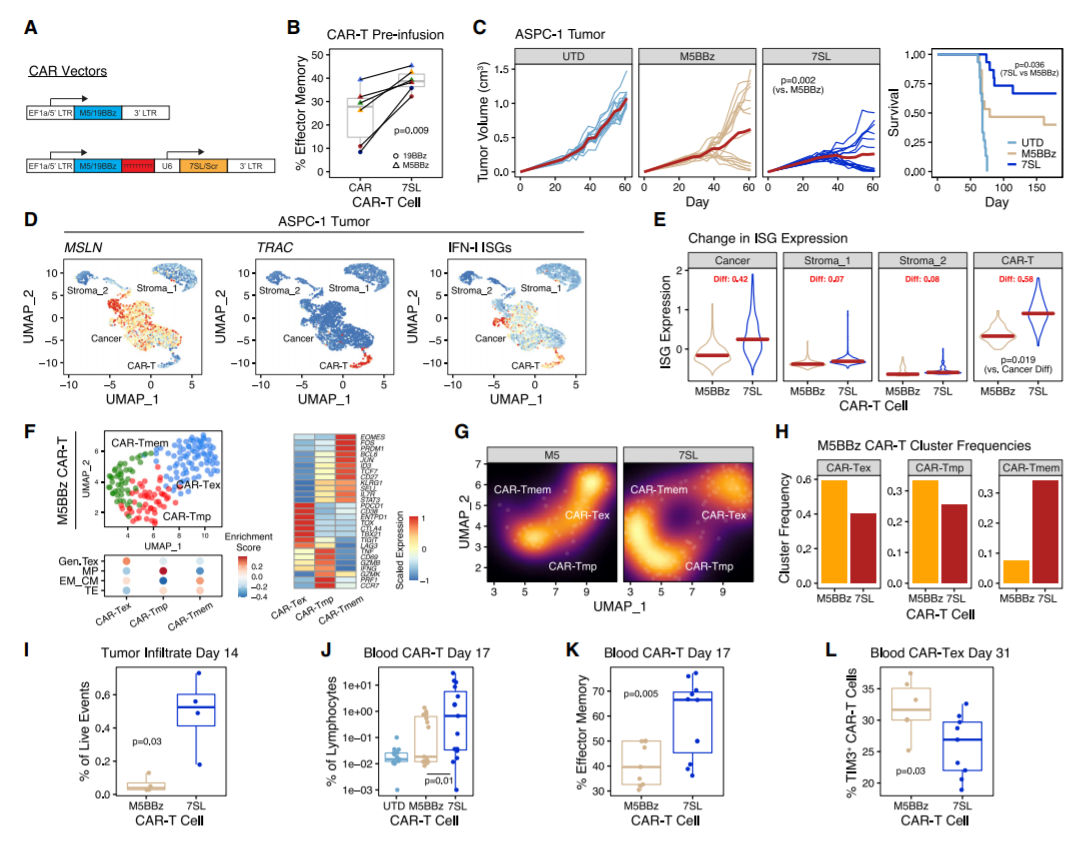
Human CAR-T cells engineered to express RN7SL1 enhance solid tumor control
The study designed CAR-T cells to deliver RN7SL1, an endogenous RNA that activates RIG-I/MDA5 signals. RN7SL1 promotes the expansion of CAR-T cells and the differentiation of effector memory. In addition, RN7SL1 is packed in extracellular vesicles and selectively transferred to immune cells. Unlike other RNA agonists, the transferred RN7SL1 restricts the development of bone marrow-derived suppressor cells (MDSC), reduces TGFB in bone marrow cells, and promotes a subset of dendritic cells (DC) with costimulatory characteristics. Therefore, endogenous effector memory and tumor-specific T cells will also expand, thereby inhibiting solid tumors with loss of CAR antigen. With the support of improved endogenous immunity, CAR-T cells can co-deploy peptide antigens with RN7SL1 to improve efficacy, even when heterologous CAR antigen tumors lack sufficient new antigens.
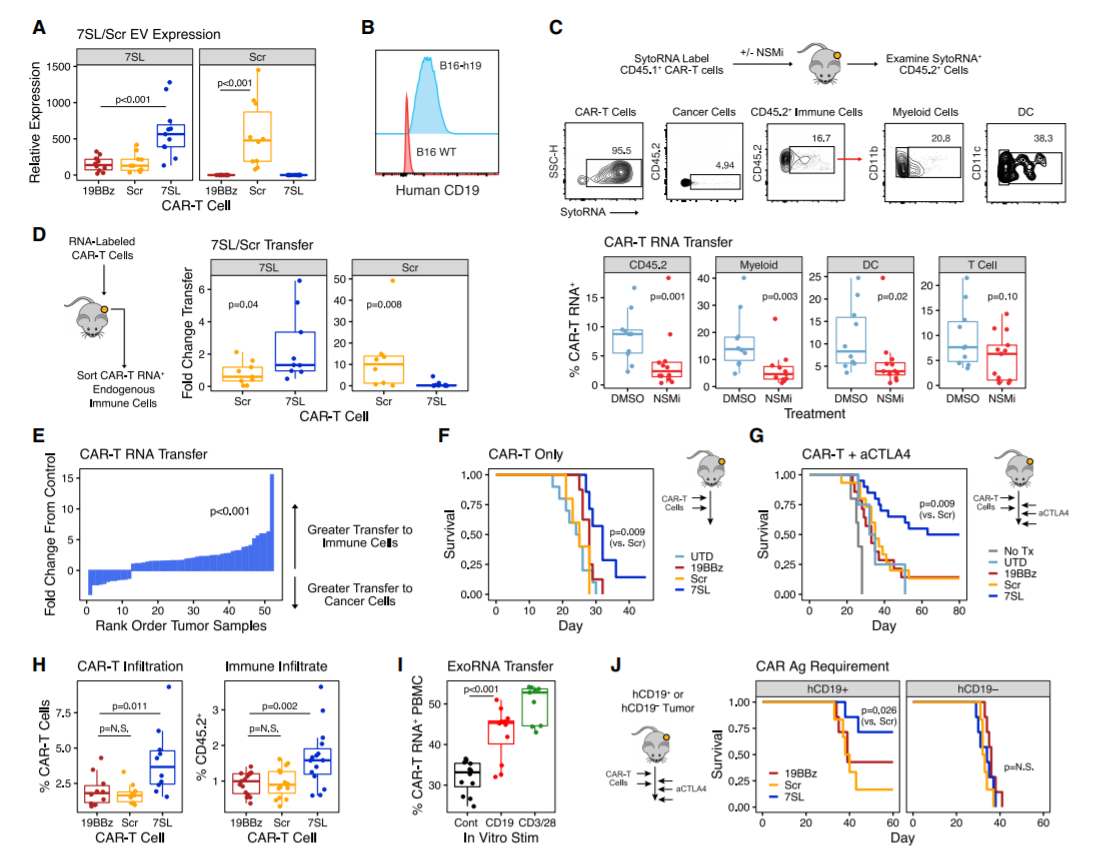
CAR-T cells deploying RN7SL1 in EVs preferentially deliver RNA to endogenous immune cells and improve anti-tumor response
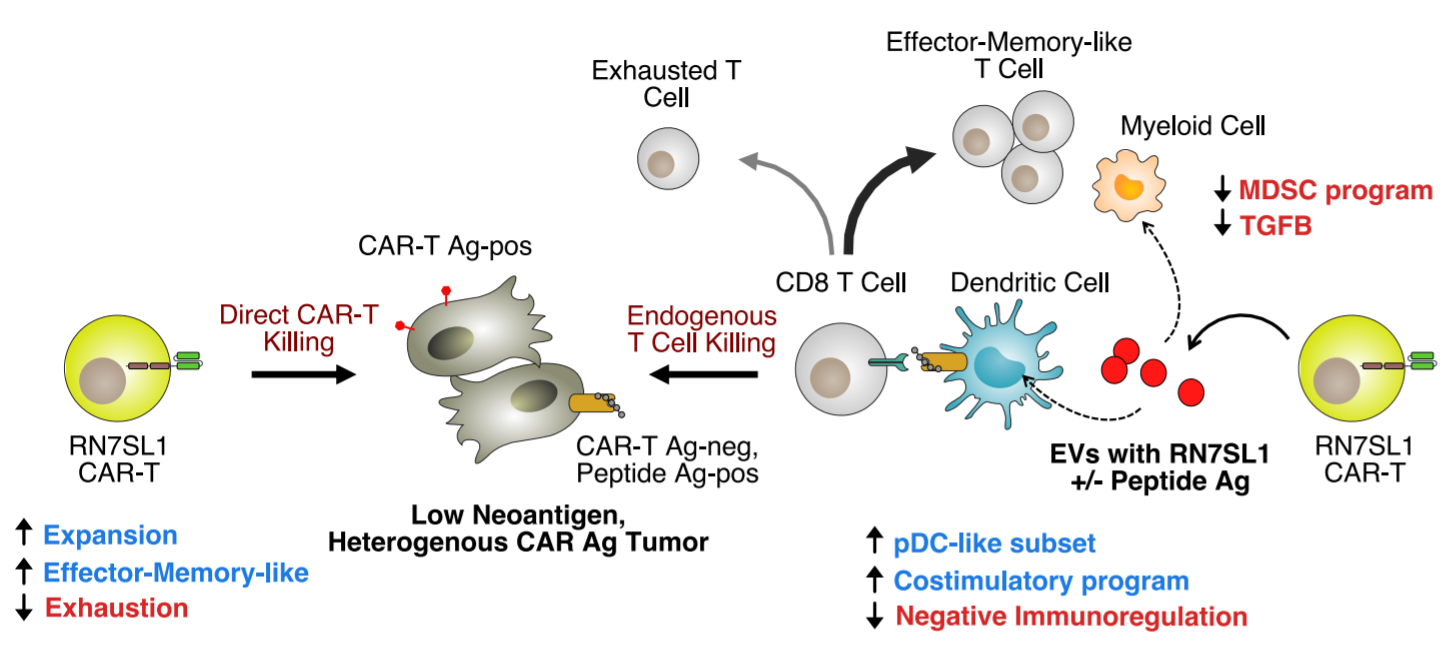
Model for how CAR-T cells delivering RN7SL1 ± peptide antigen improve solid tumor response
Main findings:
- CAR-T cells transport RN7SL1 in extracellular vesicles (EV) and activate RIG-I;
- CAR-T cells expressing RN7SL1 show better expansion and durability and less exhaustion;
- DC/myeloid cells take up EVs preferentially than tumor cells. RN7SL1 can enhance endogenous immunity;
- CAR-T EVs can deliver RN7SL1 and antigen at the same time to inhibit tumors lacking CAR antigen.
Reference: JohnsonLR, Lee DY, Eacret JS, Ye D, June CH, Minn AJ. The immunostimulatory RNARN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell. 2021 Aug 26:S0092-8674(21)00946-6. doi:10.1016/j.cell.2021.08.004. PMID: 34464586.
