Over the past few decades, viruses have been transformed into life-saving therapies, including for vaccine development and cancer treatment. The latest advancement in using viruses to treat human diseases is gene therapy—using engineered viruses as delivery vectors to replace or repair genetic defects.
Although these virus-based therapies and vaccines are game-changers, they also face numerous obstacles, especially the immunogenicity of viruses, which limits their therapeutic potential. For example, the FDA has approved several in vivo gene therapies based on adeno-associated virus (AAV), which have shown safe and effective treatment for genetic diseases. However, up to 70% of humans already have antibodies against AAV, and those without antibodies will develop them after receiving AAV gene therapy. This means AAV gene therapies can only be used once, requiring a sufficiently high dose to achieve enough therapeutic effect in one go, which significantly increases side effects at high doses. Lentivirus (LV)-based delivery vectors, on the other hand, integrate the delivered sequences into the human genome, posing a potential risk of carcinogenesis.
If we could utilize naturally occurring viruses in the human body as delivery vectors, it could address current gene therapy issues like safety risks, inability to administer repeat doses, and poor tolerance.
Ring Therapeutics, a biotechnology company incubated by the renowned venture capital firm Flagship Pioneering, has raised over $250 million, with its latest $86.5 million Series C round completed in March 2023.
Researchers at Ring discovered a diverse family of viruses called anelloviruses, which make up a significant portion of the human commensal virome. Anelloviruses have co-evolved with humans for thousands of years, stably residing within cells of various human tissues without triggering the immune system. The company aims to develop a programmable delivery platform based on anelloviruses to treat various human diseases more safely and effectively.
On March 30, 2024, Ring’s researchers published a study on the preprint platform bioRxiv titled: “A novel functional gene delivery platform based on a commensal human anellovirus demonstrates transduction in multiple tissue types.”
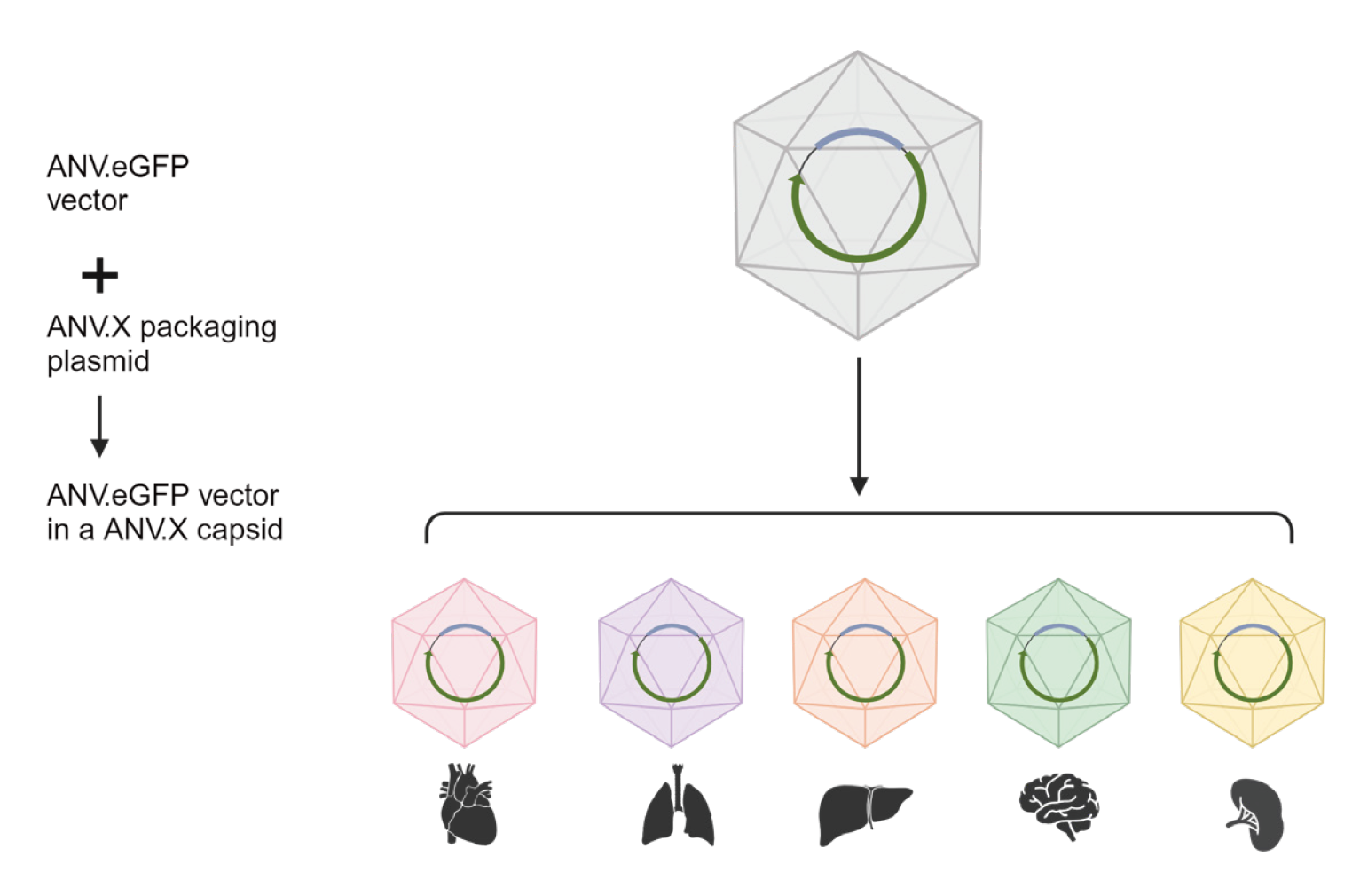
This study reported the first gene delivery vector system based on the human commensal virus—Anellovector. Anellovector showed sustained expression in mouse eyes for nine months after subretinal injection and comparable expression levels to AAV9 in the brain after intracerebroventricular injection. The newly developed viral delivery vector Anellovector has great potential to provide safe, repeatable, and effective treatments, expanding the application scope of programmable gene therapies.
Anelloviruses are non-enveloped viruses with a negative-sense, circular, single-stranded DNA genome that can infect vertebrates and are a prevalent component of the human commensal virome. Human anelloviruses can evade the humoral immune response and appear to be non-pathogenic (no association with any human disease has been identified to date). These characteristics, combined with their extensive genomic diversity and widespread tissue distribution in humans, make anelloviruses strong candidates for next-generation gene drug delivery vectors.
Ring Therapeutics is searching for new human viruses that allow multiple gene therapy treatments within a single patient, and new mouse data suggests they may have found a viable candidate.
The research team developed a new viral vector based on Ring’s proprietary human anellovirus Anellogy platform—Anellovector. Using this vector, they successfully delivered the gene for green fluorescent protein (GFP) into mouse retinas, showing stable expression for up to nine months without any signs of toxicity.
Dr. Tuyen Ong, CEO of Ring Therapeutics, stated that they have rapidly achieved the development of a novel delivery vector system based on human commensal viruses to address many challenges faced by current gene drug delivery. This latest study shows that they can successfully utilize the properties of anelloviruses to generate the viral vector—Anellovector, marking the first new viral vector in decades.
The company developed a new viral vector based on the Betatorquevirus genus of anelloviruses—Anellovector. In this study, the research team tested Anellovector’s ability to deliver the GFP gene to retinal cells. In living mice, Anellovector successfully delivered the GFP gene to retinal cells, and the expression levels were measured at three, six, and nine months. They found that although the delivered DNA copy number decreased over time, its expression levels remained stable between three to nine months without any signs of toxicity.
To see how Anellovector compared to AAV vectors and whether they worked in tissues outside the eye, the team injected a dose of either AAV9 (one of the most commonly used AAV vectors) or Anellovector into the left and right brain ventricles of two groups of mice. After 21 days, the expression levels of the Anellovector and AAV9 vectors were roughly equivalent. Brain slices showed similar expression levels between the two groups. These studies indicate that Anellovector has an affinity for cells in the central nervous system and its gene delivery efficiency is comparable to the commonly used AAV9 vector.
What We Do
Creative Biolabs provides comprehensive viral vectors and cutting-edge viral vector technology for basic research and preclinical applications, including the design and construction of suitable viral vectors and small to large-scale production of viral vectors. Our custom viral vector production stands at the forefront of gene delivery system discovery, offering a varied range of vectors that are optimized to align your project specifics while ensuring high transduction efficiency, specificity, and safety.
• Lentivirus Vector
• Adenovirus Vector
• Adeno-Associated Virus Vector
• Herpes Simplex Virus Vector
• Vaccinia Viral Vector
• Baculovirus Vector
• Alphavirus Vector
• Flavivirus Vector
• Measles Virus Vector
• Foamy Virus Vector
• Hybrid Adenoviral Vector
• Helper-Dependent Adenoviral Vector
Reference
1. Prince, Cato, et al. “A novel functional gene delivery platform based on a commensal human anellovirus demonstrates transduction in multiple tissue types.” bioRxiv (2024): 2024-03.
