Introduction: Unraveling Glycosylation’s Role in Cellular Dynamics
The enzymatic process that attaches sugar molecules to proteins or lipids extends beyond simple molecular decoration. The glycosylation process plays an essential role in cellular communication systems, pathogen defense mechanisms, and the maintenance of homeostatic balance. The glycosylation process modifies more than 70% of human proteins which positions it as a fundamental element for basic scientific research and drug development. Researchers in glycobiology need to thoroughly investigate the complexities of both n-linked glycosylation and o-linked glycosylation along with the pathological conditions that arise from these processes. The article summarizes recent discoveries along with challenges and medical possibilities in the field of glycosylation research.
1. Glycosylation Mechanisms: Precision in a Non-Templated System
The glycosylation process takes place within separate cellular compartments controlled by specific machinery.
- N-linked glycosylation:
The endoplasmic reticulum (ER) initiates this process by transferring a preassembled oligosaccharide (Glc3Man9GlcNAc2) onto asparagine residues located in the Asn-X-Ser/Thr motif. Recent cryo-EM research shows how different OST isoforms determine substrate priority during stress conditions in the glycosylation step they manage. Protein folding accuracy or degradation occurs through trimming by glucosidases I/II and ER mannosidase I. Misfolded proteins receive mannose-6-phosphate tags which designate them for ER-associated degradation through the ERAD pathway. - O-linked glycosylation:
This glycosylation pathway begins in the Golgi apparatus when polypeptide GalNAc-transferases (ppGalNAcTs) add α-GalNAc to serine or threonine residues. The enzyme C1GalT1 modifies these structures to generate diverse forms including the T-antigen (Galβ1-3GalNAc) which protects mucosal membranes and aids immune cell adhesion. O-glycans present cell-type-specific diversity because they do not follow consensus sequences unlike N-glycans.
Key Insight: The hypoxia-inducible factor 1α (HIF-1α) activates the UDP-galactose transporter SLC35A2 within tumors to increase the β1,4-galactosylation process of integrins. The modification enhances metastatic progression through improved cell-matrix binding interactions.
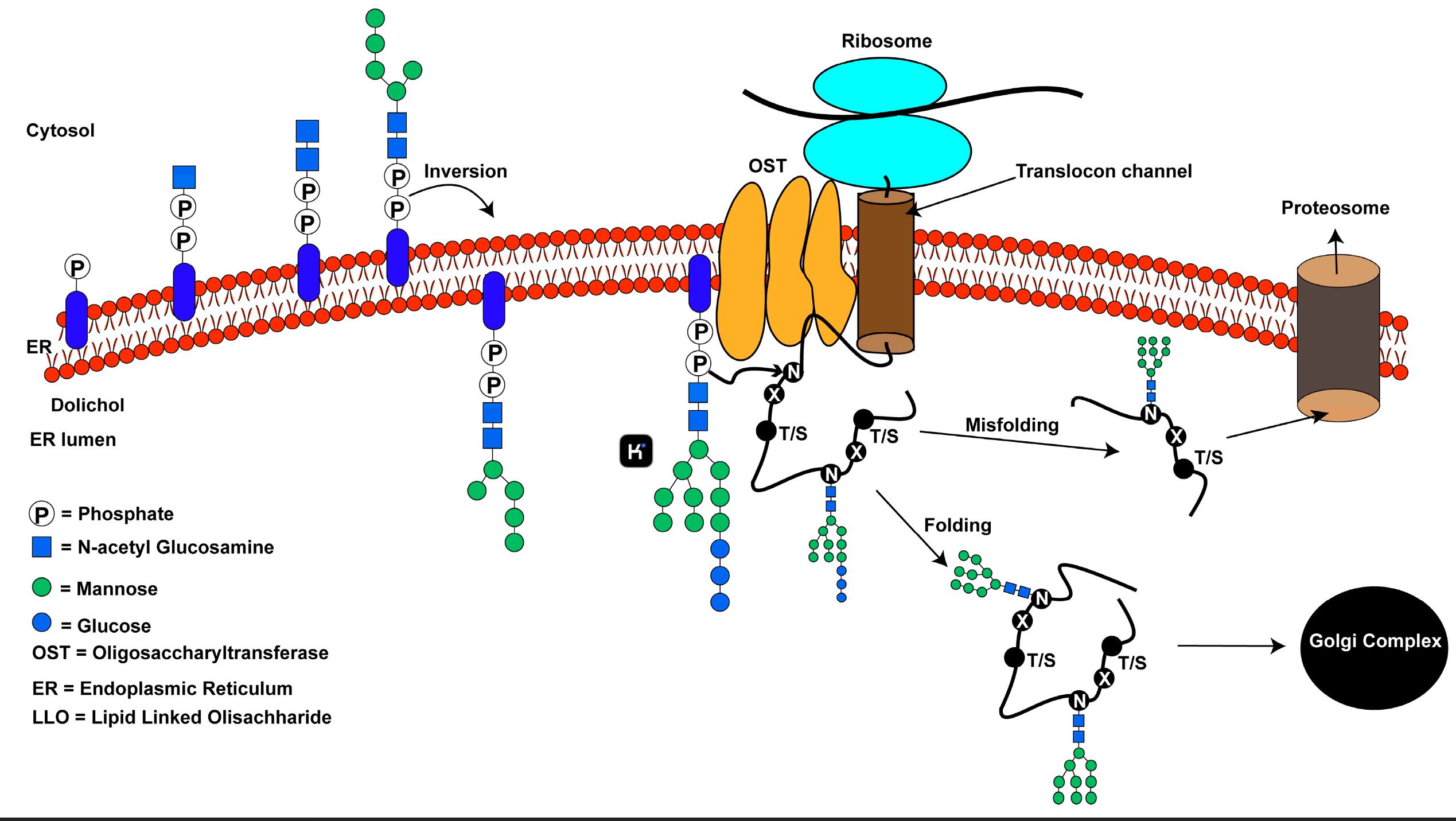
Figure 1. An overview of the N-linked glycosylation reaction of proteins in higher eukaryotes.1
2. Glycosylation Disorders: When the Sugar Code Falters
The incorrect glycosylation of proteins plays a role in multiple disease conditions.
Congenital Disorders of Glycosylation (CDGs):
Mutations in the enzyme PMM2 disrupt lipid-linked oligosaccharide biosynthesis which leads to systemic hypoglycosylation. Liver dysfunction and coagulopathies accompany neurological deficits in these patients. New treatments including oral mannose-1-phosphate supplementation seek to circumvent malfunctioning enzymatic processes according to related research.
Cancer Glycosylation:
Tumors exploit glycosylation to evade immune surveillance. ST6GAL1-mediated α2,6-sialylation of β1 integrins in pancreatic adenocarcinoma leads to the activation of FAK signaling which promotes peritoneal metastasis. Researchers found that truncated O-glycans known as Tn antigen in gastric cancer lead to chemoresistance by concealing tumor-associated antigens.
Neurodegeneration:
Alzheimer’s disease progression speeds up due to decreased tau protein O-GlcNAcylation which leads to tau hyperphosphorylation and neurofibrillary tangle development. The O-GlcNAcase inhibitor MK-8719 is currently undergoing clinical trials to achieve tau protein homeostasis.
3. Glycoengineering: Rewriting the Sugar Code for Therapy
Biologics and vaccine design are rapidly advancing due to precise control over glycosylation reactions.
1. Antibody Optimization:
CHO cells subjected to CRISPR-Cas9 mediated FUT8 knockout generate afucosylated antibodies like obinutuzumab which demonstrate improved NK cell cytotoxicity against lymphoma.
Site-specific sialylation through Chemoenzymatic remodeling with Endo-S2 decreases immunogenicity in anti-TNFα treatments.
2. Vaccine Design:
The use of recombinant HIV Env trimers with high-mannose N-glycans focuses antibody responses toward conserved epitopes while avoiding glycan shield variability. Initial testing demonstrates enhanced neutralization range capabilities.
3. Bacterial Glycosylation Systems:
Engineered E. coli strains that carry the Haemophilus influenzae glycosylation locus produce uniform glycoconjugate vaccines for pneumococcal serotypes which are currently undergoing preclinical examination.
4. Analytical Breakthroughs: Decoding the Glycoproteome
Protein glycosylation gets mapped with unmatched accuracy using state-of-the-art technologies.
- Mass Spectrometry:
New analytical methods using DIA workflows on timsTOF platforms successfully identified ST6GAL1 as a prognostic marker by examining low-abundance sialylated N-glycans in hepatocellular carcinoma exosomes. - Cryo-Electron Tomography:
Subtomogram averaging enables visualization of native glycan structures on HIV Env trimers which informs immunogen design to target exposed epitopes.
5. Challenges and Future Directions
Despite progress, critical hurdles remain:
- Structural Heterogeneity:
Stochastic glycan branching complicates glycoprotein characterization. Ion mobility-MS technology enables the differentiation of isomeric glycans which supports the quality control processes in the biosimilar manufacturing industry. - Functional Redundancy:
The functional overlap between glycosyltransferases such as ST3GAL4/6 creates challenges for validating targets. CRISPR screens performed on 3D tumor organoids reveal essential enzymes that promote metastasis. - Emerging Strategies:Glycoimmune Checkpoint Blockers:
Anti-Siglec-9 antibodies eliminate sialic acid-induced immunosuppression in glioblastoma while enhancing the effectiveness of PD-1 inhibitors.
Metabolic Glycan Labeling:
Live tumor sialylation dynamics become observable through click chemistry with azido-modified ManNAz which provides real-time insights into immune interactions.
Conclusion: Glycosylation’s Pivotal Role in Precision Medicine
Protein folding through n-linked glycosylation to immune evasion through o-linked glycosylation demonstrates glycosylation as a dynamic field for scientific exploitation. The maturity of glycoengineering platforms along with advanced analytical tools positions the field to offer groundbreaking therapies for glycosylation disorders and other areas. The combined work to chart tissue-specific glycomes with detailed enzyme kinetics analysis will solidify glycosylation’s position in precision medicine.
Advanced Glycosylation Analysis Solutions at Creative Biolabs
At Creative Biolabs, we specialize in high-precision glycosylation analysis to support your glycoprotein research and therapeutic development. Our services are designed to deliver structural insights and functional validation across diverse pathogens. Explore our categorized offerings below:
- Core Glycosylation Analysis Services
- Glycosylation Site Mapping: Detailed mapping of glycosylation sites to elucidate structural and functional characteristics.
- Virus Glycoprotein Glycosylation Analysis: Comprehensive characterization of glycosylation patterns in viral glycoproteins.
- Pathogen-Specific Glycosylation Profiling
- Coronavirus Family
- Hemorrhagic Fever Viruses
- Other Critical Pathogens
Partner with Creative Biolabs to leverage cutting-edge glycosylation analysis technologies and accelerate your research on vaccine development, antiviral therapies, and structural biology. Our expert team ensures reliable data and tailored solutions for your unique challenges.
Contact us today to optimize your glycoprotein characterization workflows with industry-leading precision. Let’s advance your discoveries together!
Reference:
Mohanty S, Chaudhary BP, Zoetewey D. Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyl transferase. Biomolecules. 2020 Apr 17;10(4):624. https://doi.org/10.3390/biom10040624. Distributed under Open Access license CC BY 4.0, without modification.
