Therapeutic Antibody Drugs
Since the first clinical application approval in 1986, more than 100 therapeutic antibody drugs have entered clinical application successively, and new antibody drugs are in the process of development and marketing every year.
With the development of technology, common technologies for antibody screening and discovery include phage display technology, transgenic mouse hybridoma technology, and single B cell technology. Phage display technology realizes the unification of protein or polypeptide genotype and phenotype, and the required antibody can be quickly screened by using existing gene fragments, but it is highly dependent on the library and cannot cope with the diversity and complexity of antibody types. The transgenic mouse hybridoma technology does not need to go through the traditional steps of expressing recombinant antibodies in molecular biology. It only needs to go through animal immunization, cell culture and cell fusion, mouse ascites preparation, and monoclonal antibody purification to complete the preparation of target antibodies. However, it still has the disadvantages of complicated operation, high difficulty, and a low success rate. Single B cell technology has the advantages of high efficiency, complete human origin, and rich gene diversity. However, so far, the application potential of single B cell antibody production technology has not been realized. Single B cell antibody production technology is still limited by some practical conditions. Antibody discovery technology relying on protein de novo sequencing is simple and quick to operate, does not rely on any antibody library, and can handle a variety of antibody detections, so it has attracted more and more attention and favor from antibody drug discovery and developers.
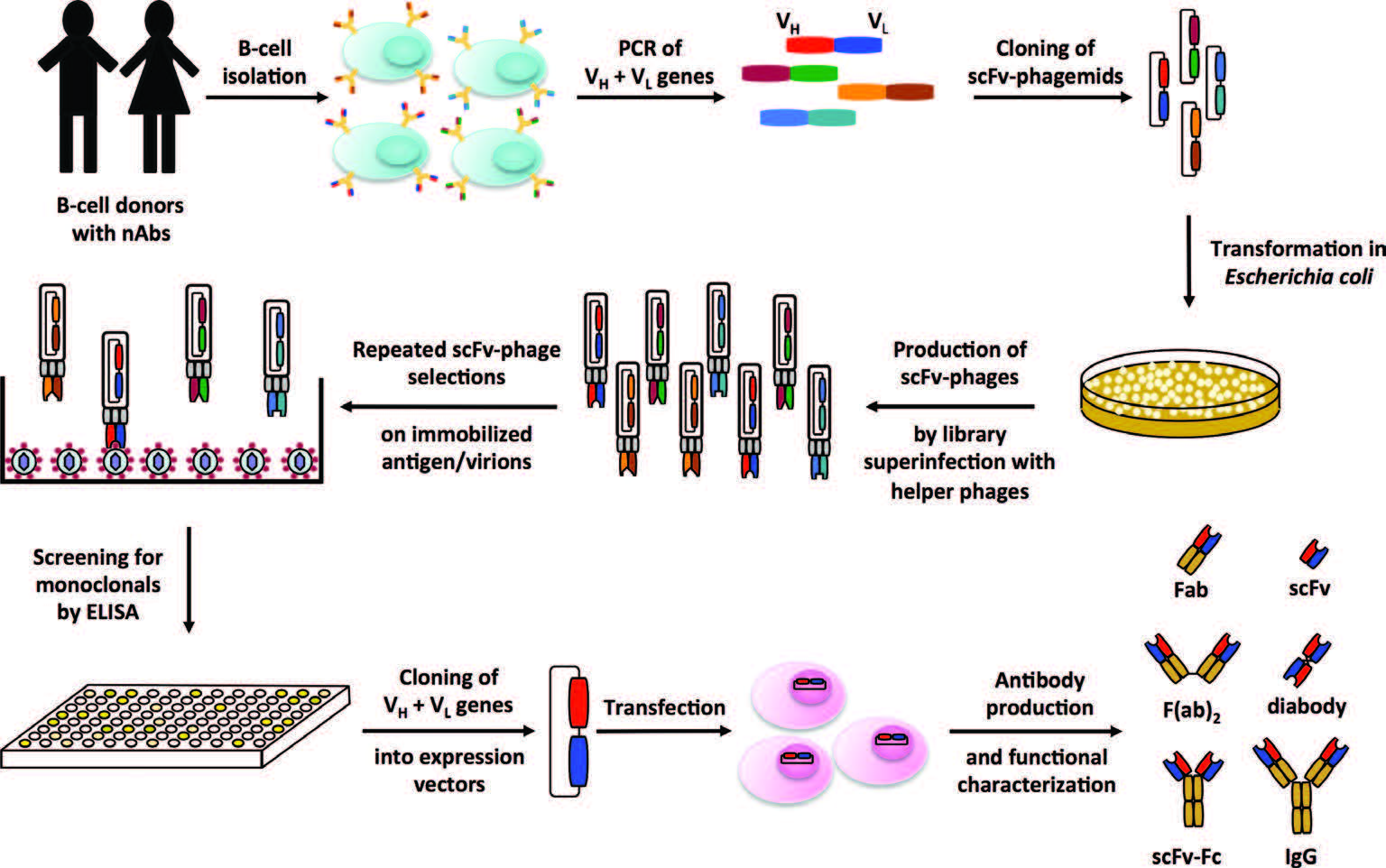
Figure 1. Screening of monoclonal antibodies by phage display technology (Philipp Diebolder, 2017)
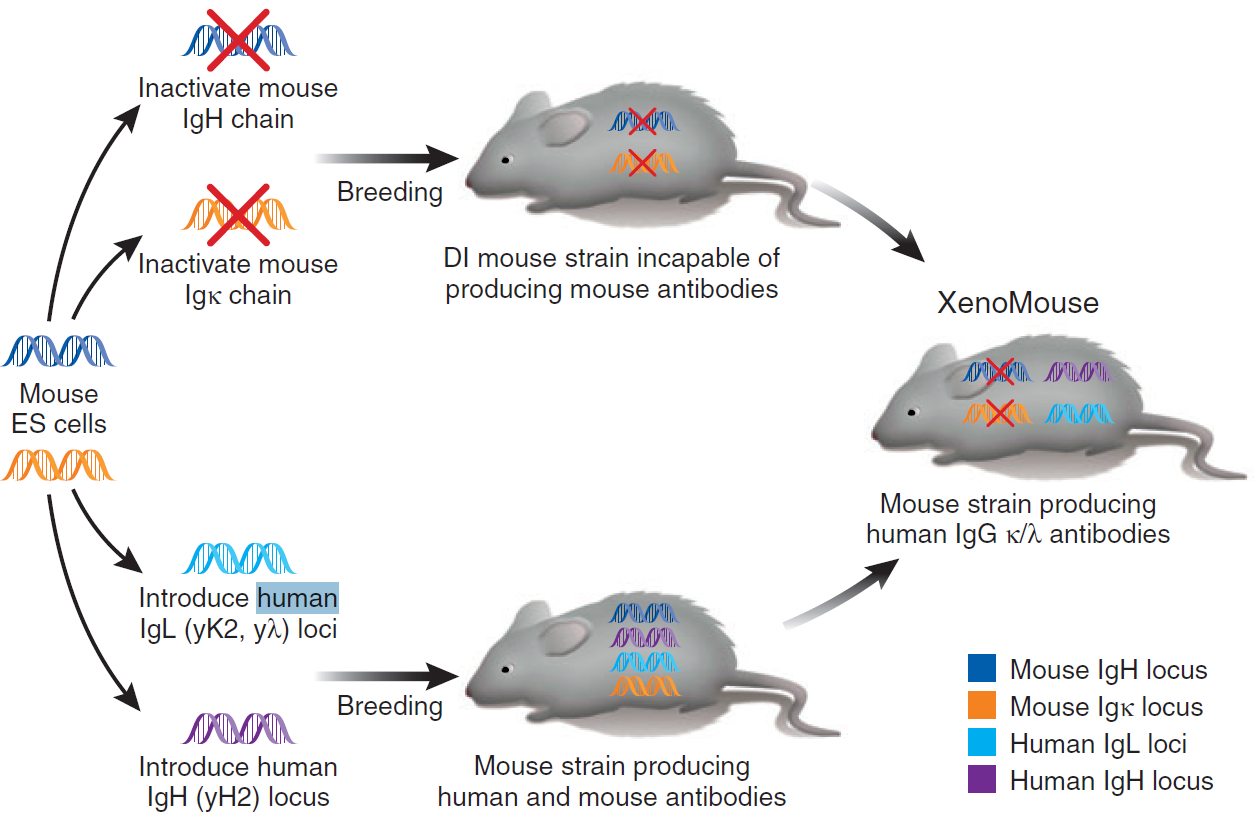
Figure 2. Transgenic mouse hybridoma technology (Aya Jakobovits, 2007)
Protein Sequencing
The discovery of antibody sequences relies on gene and protein sequencing. Compared with gene sequencing, protein sequencing produces far fewer random matches, which is more conducive to the discovery of new antibody sequences. Among the protein sequencing methods, there are currently three commonly used methods: sequencing based on the Edman degradation method, peptide mapping analysis, and protein de novo sequencing. Among them, protein de novo sequencing has the advantage of being able to sequence the full length of any unknown protein without relying on any protein database, so it has become the preferred technology to obtain protein sequences.
At present, the antibody sequencing technology developed based on protein de novo sequencing has gradually attracted the attention of researchers including basic research and antibody drug development. Creative Biolabs’ antibody discovery platform can directly and efficiently discover high-affinity natural antibodies that play a role in the immune system, facilitate the production of recombinant antibodies, and provide a new way for antibody research and development.
Creative Biolabs’ protein de novo sequencing technology breaks through the limitation of samples, can obtain protein sequences in complex samples, breaks the database dependence of traditional proteomics, and truly realizes de novo protein sequencing. Among them, Creative Biolabs has continuously optimized and upgraded the antibody sequencing technology based on its 20 years of technical accumulation, and developed a simpler, faster, and more accurate antibody sequencing platform. The technical advantages of Creative Biolabs’ protein de novo sequencing technology platform are as follows:
- Easier to break through sample limitations
- Faster, high-throughput sequencing to obtain information
- More rigorous, 100% guaranteed sequence accuracy
Application Scenarios of Creative Biolabs Antibody Discovery Platform
Based on the above characteristics and advantages, Creative Biolabs’ antibody discovery service has been fully applied in many fields. These practical applications further highlight the professionalism and industry leadership of Creative Biolabs in antibody discovery.
- New natural antibody discovery in tumors
- Natural antibody production from the patient’s own source
- Research on therapeutic antibodies for autoimmune diseases
- Identification of the immune response after intervention
- Conversion of polyclonal antibodies to monoclonal antibodies or monoclonal antibody mixtures
Reference
Smith SL. Ten years of Orthoclone OKT3 (muromonab-CD3): a review. J Transpl Coord. 1996 Sep;6(3):109-19; quiz 120-1. doi: 10.7182/prtr.1.6.3.8145l3u185493182. PMID: 9188368.
Diebolder, P., & Krawczyk, A. (2018). Detailed Protocols for the Selection of Antiviral Human Antibodies from Combinatorial Immune Phage Display Libraries. InTech. doi: 10.5772/intechopen.70139.
Janin-Bussat MC, et al. Characterization of antibody drug conjugate positional isomers at cysteine residues by peptide mapping LC-MS analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Feb 15;981-982:9-13. doi: 10.1016/j.jchromb.