In a study published in Nature, scientists from St. Jude’s Children’s Research Hospital and other institutions have developed a new treatment strategy to effectively enhance cancer immunotherapy, which may effectively slow tumor growth and prolong the life of cancer mice. The findings of this study may provide a promising strategy for the development of a more effective adoptive cell therapy, such as CAR T cell therapy. Immunotherapy aims to use the patient’s own tumor-specific T cells for cancer treatment. Before these T cells are reinjected into the patient’s body, the researchers will collect them, expand their function, and so on; when reinjected into the patient’s body, some patients will have a significant response to the treatment. However, adoptive cell therapy may not be effective against solid tumors.
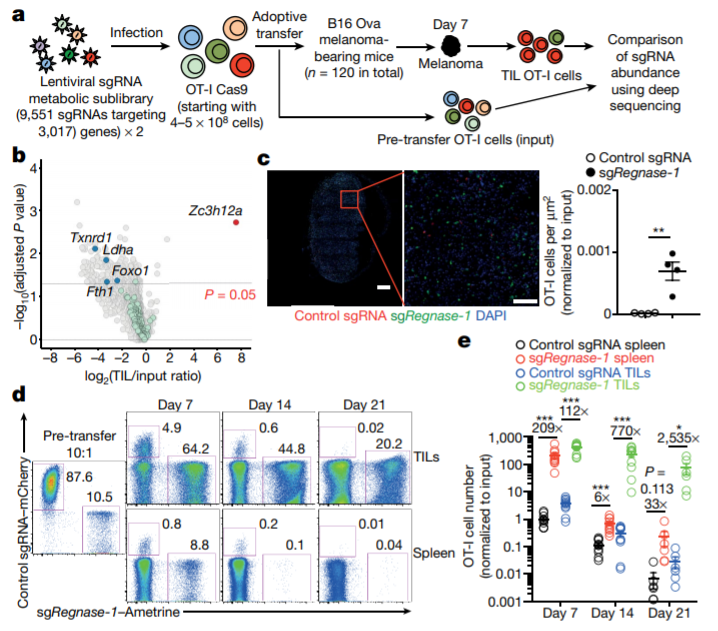
In vivo CRISPR-Cas9 screening identifies REGNASE-1 as a major negative regulator of the antitumor responses of CD8+ T cells. (Jun Wei et al. Nature, 2019)
“Our goal is to increase the persistence of tumor-specific T cells and their anti-tumor efficiency,” said researcher Hongbo Chi. “This study may provide us with a way to reprogram tumor-specific T cells to be as persistent as long-lived primitive or memory T cells, while also showing strong killing activity like normally functioning effector T cells.”
In this paper, the researchers used CRISPR-Cas9 technology to identify a special enzyme in tumor-specific T cells, which is similar to a “brake” that could turn off the body’s anti-tumor immune response. When the enzyme, called REGNASE-1, is removed, T cells live longer, and their efficacy and accumulation in tumors increase. When REGNASE-1-deficient T cells are used to treat leukemia and melanoma mice, they live longer and have smaller tumors than those treated with conventional T-cell (wild type) therapy.
“Previous researchers have found that REGNASE-1 can limit T cell activation, but in this study, we found that REGNASE-1 can also inhibit two important T cell signaling pathways,” said researcher Jun Wei. They used CRISPR-Cas9 for screening and found that transcription factors BATF and TCF-1 might be targets for REGNASE-1. At the same time, scientists point out that BATF can drive the metabolism of T cells to enhance the ability of T cells to kill tumor cells, while TCF-1 can promote the longevity of T cells. The traditional view is that these processes interact with each other and increasing the anti-tumor activity of T cells means that T cells live longer, but the results of this study found that this may not be the case in fact.
The researchers said that combination therapy may be the key to the clinical success of cancer immunotherapy. To this end, they also want to provide more insights into the clinical potential of the findings. After a second CRISPR-Cas9 screening, the researchers identified two more related molecules, and when these two molecules (signaling factors PTPN2 and SOCS1) were removed along with REGNASE-1, the performance of T cells in cancer immunotherapy was significantly improved. Therefore, PTPN2 and SOCS1 may not depend on REGNASE-1 to play a role. Later, researchers will conduct more in-depth studies to clarify how to target REGNASE-1 to develop new anti-cancer therapies.