On March 29, 2017, Novartis announced that the US FDA has issued a priority review for its CAR-T therapy CTL019 (tisagenlecleucel-T) biologics licensing application. The indications of CTL019 was recurrent or refractory B-cell acute lymphoblastic leukemia in children or young people.
In the past two years, Immune cell therapy caused a great sensation in the field of cancer treatment. Especially immunotherapy based on CAR-T technology brought hope for cancer patients. At present, the world’s CAR-T clinical trials most used CD19 as target, and the products against CD19 molecule target also achieved remarkable results.
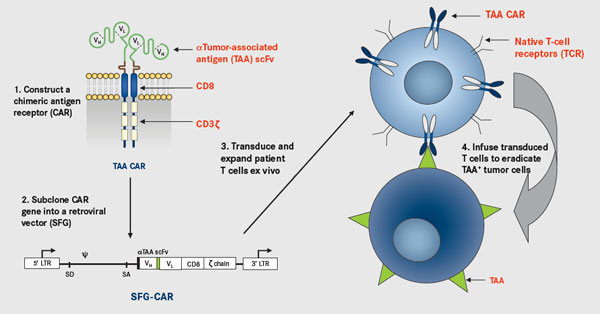
Among the research institutions carrying out CAR-T technology clinical trials in the world, KITE and Novartis would be the leading place, and the first batch of CAR-T products mostly came from these two companies. Earlier reports said that KITE had filed a listing application with the company’s CAR-T therapy KTE-C19 for three more malignant non-Hodgkin’s lymphoma (NHL): diffuse large B-cell lymphoma (DLBCL), metamorphic follicular lymphoma (TFL) and primary mediastinal B-cell lymphoma (PMBCL). Novartis was not far behind. They plans to complete their own CAR-T therapy CTL019 products listed on the application, and the products are mainly used in the recurrence of refractory acute lymphoblastic leukemia (rrALL). Juno, as the most promising company before, was eventually stopped because of frequent clinical accidents on JCAR015 (ROCKET).
Kite Pharma’s CAR-T cell therapy formulation KTE-C19 for non-Hodgkin’s lymphoma is expected to be available in 2017, based on the results of the trial and the feedback given by the FDA. If it goes well, Kite Pharma is expected to be the first company to introduce commercial cell therapy products before Novartis.
In December 2016, Kite submitted the first CAR-T therapy new drug application. “Kite Pharmaceutical CAR-T therapy KET-C19 will be the first to hit the market,” overwhelming media reported. However, the fact is not as good as Kite expects. On March 29, 2017, Novartis announced that the US FDA has issued a priority review for its CAR-T therapy CTL019 (tisagenlecleucel-T) biologics licensing application. The indications of CTL019 was recurrent or refractory acute B-cell acute lymphoblastic leukemia in children or young people. Now, Novartis is more likely to achieve the “first dose.”
In December 2016, the 58th annual meeting of the American Society of Hematology hosted by the American Society of Hematology (ASH) was held in San Diego, USA. At the meeting, Kite and Novartis published the results of CAR-T therapy, respectively:
Kite had published the Phase I clinical trial data of ZUMA-3 and ZUMA-4 from CAR-T cell immunotherapy KTE-C19. ZUMA-3 and ZUMA-4 clinical studies were directed to adult and children with recurrence/refractory acute lymphoblastic leukemia. Of the 11 patients, 9 patients had complete remission, with a response rate of 82%, and no residual lesion was detected in patients with complete remission. Five of these patients in Kite suffered from grade-3 or more cytokine release syndrome; five patients suffered from grade-3 or more central nervous system complications. Moreover, one patient died of KTE-C19-associated cytokine release syndrome in the ZUMA-3 treatment test; in the ZUMA-4 treatment test, one patient died of disseminated fungal infection independent of KTE-C19.
Novartis also published mid-term analysis data after treatment (between 3-23 years old). The data showed that three months after the use of CTL019, 41 of the 50 patients had complete remission with a response rate of 82%. The lesion was not detected in all patients with complete remission, and the overall survival rate was 89% within 6 months of CTL019, 60% of which had no recurrence. In terms of safety, 48% of patients suffered from grade-3 (severe) to grade-4 (life-threatening) cytokine release syndrome (CRS), but none of the deaths occurred. 15% of patients suffered from grade-3 central nervous system complications, including encephalopathy and conception. There were no grade-4 and above central nervous system complications.
It is well known that the efficacy and safety of a new clinical drug must be validated in clinical trials before it can be approved by regulatory authorities. These clinical trials are usually randomized, double-blind and controlled trials with sufficient participants. For example, Kite is a biopharmaceutical company with CAR-T therapy as the main asset, and any rumors related to clinical trials will seriously affect its stock market performance. More importantly, early disclosure of immature experimental results will also affect the rigor of clinical trials. Previously, the FDA had questioned the reliability of the trial due to the early disclosure of incomplete clinical trial results. Although the KTE-C19 experiment is a multi-center experiment with a single arm and open label, the death of a patient during treatment is a major event in clinical trials both for patients and for pharmaceuticals. There seems has been no death case exposure in Novartis. So considering the safety, it must be Noble to win. CAR-T cells in Kite’s ZUMA clinical trial are distributed in 23 different locations, but are limited to be used in the United States. In contrast, Novartis’s ELIANA clinical trial is the first of a globalized CAR-T product. It is shipped to 25 hospitals in North America, Europe and Asia Pacific.
Anyway, regardless of the results of the two giants, CAR-T’s era is really coming!
