The Antibodies are secreted by plasma cells transformed from B cells, and each B cell line can produce only one specific antigenic determinant. The antibody, produced from a single cell line, is called the monoclonal antibody (McAb). The first generation of monoclonal antibodies came from hybridoma antibody technology developed by Koehler and Milstein in 1975. On the basis of cell fusion technology, mouse B cells capable of secreting specific antibodies and mouse myeloma cells with unlimited reproductive ability were fused into B cell hybridoma. A specific antibody against an antigenic epitope can be prepared by culturing a group of cells with a single hybridoma cell with this property, as shown in figure 1. However, the human immune system can recognize mouse monoclonal antibodies, which can cause human anti-mouse antibody (HAMA) response. This not only shortens the half-life and weakens the efficacy of therapeutic monoclonal antibodies, but also sometimes causes serious adverse reactions, so the clinical application of the first generation of monoclonal antibodies is greatly limited.
Figure 1. Illustration showing the production route of hybridoma technology.
Since the advent of the first mouse monoclonal antibody Muromonab OKT3 in the world in 1986, nearly 80 monoclonal antibodies have been on the market in the world. So far, the monoclonal antibody has developed to the fourth generation: the first generation is mouse monoclonal antibody (momab), the second generation is human-mouse chimeric monoclonal antibody (ximab), the third generation is humanized monoclonal antibody (zumab), the fourth generation is fully human monoclonal antibody (mumab). The advantage of humanized monoclonal antibody and human monoclonal antibody is that it can overcome the reaction of human anti-mouse antibody, prevent the monoclonal antibody molecule from being quickly eliminated by the immune system as heterogenous protein, and improve the biological activity of monoclonal antibody molecule. In particular, the variable and constant regions of human antibodies are human, which can further remove immunogenicity and side effects on the basis of humanized antibodies. Humanized antibodies and human antibodies have the characteristics of high affinity, high specificity and low toxicity and side effects, which greatly overcome the shortcomings of mouse antibodies and chimeric monoclonal antibodies. Therefore, it has become the inevitable trend of the development of therapeutic antibody drugs.
Monoclonal antibodies usually target disease-related antigens or specific receptors on the cell surface, such as the PD-1 receptor on the surface of tumor cells and the PD-L1 ligand on the surface of T cells. PD-1/PD-L1 inhibitors, which are in the limelight, belong to monoclonal antibodies and are the focus of tumor immunotherapy in recent years. Nivolumab and pembrolizumab, which are on the market, are PD-1 inhibitors and are mainly used in the treatment of melanoma and non-small cell lung cancer. PD-L1 inhibitors atezolizumab (trade name Tecentriq), durvalumab (trade name Imfinzi) and avelumab (trade name Bavencio) have been approved for the treatment of urethral epithelial cancer. On September 28, 2018, FDA approved the listing of Libtayo (cemiplimab-rwlc) jointly developed by Sanofi (Sanofi) and Regenerative. It is used to treat metastatic skin squamous cell carcinoma (CSCC) or locally advanced CSCC patients who cannot receive healing surgery or radiotherapy. This is also the third anti-PD-1 antibody approved by FDA.
Figure 2. Mechanism of PD-1 receptor and PD-L1/L2 inhibitors mediated cancer immunotherapy.
Up to now, monoclonal antibody drugs have become an important part of biomedicine and have broad application prospects in medical treatment. It has been successfully used in the treatment of tumors, autoimmune diseases, infectious diseases, transplantation rejection and other diseases. However, there are many bottlenecks in the preparation of monoclonal antibodies. At present, the bottleneck of antibody drug research and development lies in the screening of target molecules, humanization of antibodies and preparation of human antibodies, high-throughput and large-scale screening of antibodies, prediction, modeling and analysis of antigenic epitopes, construction of three-dimensional configuration of antigen-antibody interaction and various techniques to increase the function of antibody effect.
1.Target Screening of Antibody Drug
Traditional antibody drugs are developed at the level of a single gene, a single protein, and a single antibody. First of all, it will take many years to study the function of the gene and its coding protein to confirm whether the gene and its encoded protein can be used as antibody drug targets to develop antibody drugs. The main drawback of this method is that the number of antibody drug targets obtained is extremely limited, and these targets were discovered more than a decade ago and it takes a long time, usually 10 to 20 years. With the continuous progress of genomics, transcriptome, proteomics and sequencing technology, more and more new genes and proteins have been found, which is expected to select suitable antibody drug targets.
What are the criteria for screening antibody drug targets? In the case of antineoplastic drug targets, first of all, there should be differences in target expression, such as differences between normal and tumor tissues, or loss of expression in key host organs, or persistent expression in the progress of the disease. Second, the target should play a role, when the use of antibodies for treatment, the antigen cannot be easily degraded by enzymes. The production of high affinity antibodies from known therapeutic targets is not a major obstacle, but the main challenge is to screen target molecules.
Now, scientists have used humanized antibody technology and fully human antibody technology, hoping to find some better antibody targets through the human immune system, so as to develop better antibody drugs. The fully human antibody technology is optimized by human-mouse hybridoma technology, human-human hybridoma technology, B cell immortalization and high-efficiency and high-throughput fully human antibody library technology. The selection, maturation and production of antibodies are all formed in the human body, so they are all human antibodies in the strict sense. Antibody targeting, antibody production and post-transcriptional modification are all completed by the human immune system after a series of screening. The antibodies produced by this technique have the best natural affinity and binding power, and act more effectively on the human body. High-efficiency and high-throughput fully human antibody library technology will be able to secrete antibodies of the target cell isolation, purification, enrichment and proliferation. The specificity of the antibody secreted by B cell subclone can be screened and identified by ELISPOT, ELISA or hemolytic plaque test. The gene sequence of the target antibody was obtained from the monoclonal cultured cell line, and the prokaryotic or eukaryotic expression vector was constructed and transferred into engineering bacteria or cells to reconstruct the activity of the antibody.
2.Immunogenicity Analysis of Monoclonal Antibody Drugs
At present, the common adverse reactions of monoclonal antibodies are mainly caused by their immunogenicity. The anti-drug antibodies caused by immunogenicity have a great influence on the safety and efficacy of the drug. Immunogenicity is one of the decisive factors in the development of biotech drugs, so their immunogenicity should be taken into account when evaluating drug safety. To this end, scientists have taken measures to improve the immunogenicity of monoclonal antibody drugs, such as humanization of antibodies, improvement of solubility, protein modification (such as protein polyethylene glycol modification) and improvement of effector molecule function.
Figure 3. Polyethylene glycol modification of antibodies.
The current methods for evaluating and analyzing the immunogenicity of monoclonal antibodies include enzyme-linked immunosorbent assay (Elisa), liquid chromatography–mass spectrometry (LC-MS), surface plasma resonance (SPR), electrochemiluminescence (ECL) and radioimmunoassay (RIA). However, these methods have not yet reached a unified conclusion on the critical value of immunogenicity, and the critical value of immunogenicity is different due to different distribution laws and calculation companies, which makes it impossible to unify the acceptance criteria among different drugs. However, with the continuous progress of molecular biology technology, the humanized components of monoclonal antibodies have been improved, and even the whole human antibodies have been reached. Improving the binding and effector molecular function of these antibodies, combined with protein modification, is expected to avoid the immunogenicity of monoclonal antibodies. At the same time, improving the immunogenicity detection method, unifying and standardizing it will make the clinical trial have clear guiding principles, and finally accelerate the clinical application of monoclonal antibody drugs.
3.High-throughput Animal Cell Expression Technique
In terms of protein expression system, in recent years, scientists have developed and optimized the expression system of many antibody molecules, such as bacteria, yeast, insect cells, mammal cells, plant cell expression systems and in vitro expression systems. Among them, mammalian cell expression system has many important advantages, such as high activity and good stability, and has become the most important expression system in the manufacture of antibody biotechnology products.
From the point of view of the scale, speed and function of antibody preparation, the development of high-throughput antibody preparation technology is very important. Large-scale and efficient culture of mammal cells is the main mode of production and key bottleneck technology of biomedical products. At present, there are flow culture technology and perfusion culture technology in the world.
4.Construction & Optimization of Humanized and Fully Human Antibodies.
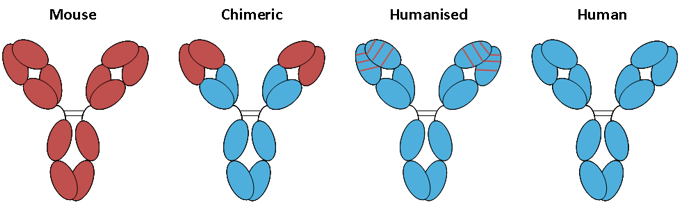
With the development of immunology and molecular biology, DNA recombination technology is more and more used in antibody construction and optimization. The techniques for the construction and optimization of humanized antibodies include resurfacing antibody and reshaped antibody. Resurfacing antibody refers to the humanization of amino acid residues on the surface of heterogenous antibodies. The principle of this method is to replace only the regions which are obviously different from the surface of human antibody, and to replace amino acids similar to the surface residues of human antibody on the basis of maintaining antibody activity and reducing heterogenicity. In addition, there should not be too many segments to be replaced, and the residues that could affect the size of the side chain, charge, hydrophobicity, or may form hydrogen bonds, which influence the conformation of the complementary determining region (CDR) of antibody, should not be replaced as far as possible. The reshaped antibody refers to the antibody constructed by the splicing of the antigen-binding residues of the heterogenous antibody with the human antibody, including complementary determining region transplantation, partial complement determining region transplantation and specific determining region transplantation.
Figure 4. Chimeric antibodies and humanized antibodies.
Both light and heavy chains of humanized antibodies come from human beings and are the development region of therapeutic antibodies. At present, there are antibody library screening techniques for the construction and optimization of humanized antibodies, such as chain replacement and genetic engineering mice to prepare humanized antibodies. The more mature antibody library screening techniques include phage antibody library, synthetic antibody library and ribosome display technology.
Antibodomics technology is based on genomics and proteomics, combined with hybridoma technology and genetic engineering antibody technology, after high-throughput screening of antibody targets, the establishment of large-scale antibody library, and finally applied. Compared with the traditional monoclonal antibody technology, the antibody library technology has the advantages of large library capacity, more species screening, easier to obtain highly active monoclonal antibodies against specific antigen epitopes and so on. At the same time, the antibody library technology is more timesaving, labor-saving, efficient and economical in the screening process.
Mice are still the easiest animal species for immunization and subsequent genetic engineering, but the mouse antibody V region gene is still obtained through the mouse antibody library. In order to make it safe for clinical use, follow-up humanized transformation must be carried out. The transgenic mouse technology of fully human antibody developed in the past two years enables us to prepare a human immune antibody library through transgenic mice with a complete set of human antibody genes, from which we can directly screen the V region gene of the fully human antibody with therapeutic value. There is no need for humanized transformation.
5.Development of New Antibody Drugs
Traditional antibody drugs inhibit tumor growth by blocking a single signal pathway, and the drug resistance of antibody drugs is easy to appear in clinic. Therefore, bispecific antibody (BsAb) came into being. By means of genetic engineering, two antibody fragments targeting different antigens are combined together, which has two antigen binding sites, which can play a synergistic role and improve the therapeutic effect. This structural design can effectively improve the pharmacokinetic process of antibody drugs in vivo and enhance the clinical therapeutic effect. However, the design of BsAb with good curative effect, high stability and conducive to production still needs to be further studied.
The antibody drugs on the market in recent years reflect the new trend of the development of the next generation of antibody drugs. The first direction is to make antibody drugs have a smaller molecular weight, so that they have better pharmacokinetic and pharmacodynamic parameters, and are easier to manufacture on a large scale, such as Fab fragment, Fab’ fragment, F(ab’)2 fragment, Single-chain variable fragment (scFv), single domain antibody (sdAb), diabody, triabody and minibody. The second direction is to connect at least two molecules with certain biological functions to form fusion proteins based on known drug molecules, such as bispecific antibody, trifunctional antibody, synthetic antibody (synbody), antibody-drug conjugate (ADC).
Antibody-drug couplers are composed of monoclonal antibodies and small molecular drugs with therapeutic effect. These drugs realize the targeted delivery of chemical drugs to tumor tissue with the help of antibodies. The antibody-drug conjugate has high stability in the blood, and the drug molecules will not fall off, so the toxic and side effects are small, but the inhibitory effect on tumor cells is much higher than that of naked antibodies. This design strategy can not only improve the killing ability of antibody drugs, but also improve the treatment window of small molecular chemicals.
Figure 5. Antibody-drug conjugate.
Monoclonal antibody drugs provide a new way for the treatment of a variety of diseases. At present, monoclonal antibody drugs have been widely used in the clinical treatment of tumors and other diseases. From the perspective of anti-tumor monoclonal antibody drug development process, it is mainly divided into five parts: target discovery, target selection, antigen preparation, selection of monoclonal antibody preparation technology and antibody function identification. Through the technical characteristics of these links, we can find the risk factors that affect the R&D results, find the risk factors, and use the thinking and methods of risk management to analyze. Different technologies used in the preparation of monoclonal antibodies will encounter different challenges. For this reason, it is necessary for developers to carry out specific analysis of specific problems and constantly overcome these technical challenges in order to develop truly useful monoclonal antibodies and bring new life-saving drugs to patients.