Recently, a research paper entitled “Structural insights into the membrane microdomain organization by SPFH family proteins” was published in Cell Research. The high-resolution structure of super membrane complexes formed by two representative SPFH family protein molecules (HflK and HflC) and AAA+ protease FtsH located in bacterial intima was studied by cryo-electron microscopy (Cryo-EM), which revealed the common principle and molecular basis of SPFH family proteins in different membrane microdomain organizations.
The composition of cell membrane and the distribution of lipids and proteins are highly heterogeneous. A common feature of the cell membrane systems of prokaryotes and eukaryotes is that they both have relatively discrete functional membrane microdomains (FMMs). However, none of the roles of SPFH family proteins, either in general FMM scaffolding or in specialized regulatory functions, is understood at molecular level. SPFH (Stomatin, Prohibitin, Flotillin, and HflK/C) family proteins are enriched in prokaryotic and eukaryotic FMMs of various subcellular localizations, including plasma membrane, nucleus, Golgi apparatus, endoplasmic reticulum (ER), endosomes, lipid droplets, and mitochondria. SPFH family proteins are iconic molecules in FMM, which are speculated to play a basic role as scaffolds in the formation and organization of FMM based on their oligomeric properties. HflK and HflC are two kinds of SPFH membrane proteins which exist widely in bacteria. They are bacterial homologous proteins of mitochondrial prohibitins, which can form a super complex with AAA+ protease FtsH (HflK-HflC-FtsH, referred to as KCF complex) anchored in the bacterial inner membrane to regulate the quality control and homeostasis regulation of membrane proteins. In this study, the composition and high-resolution structure of KCF complex were studied by cryo-electron microscopy 3D reconstruction technique.
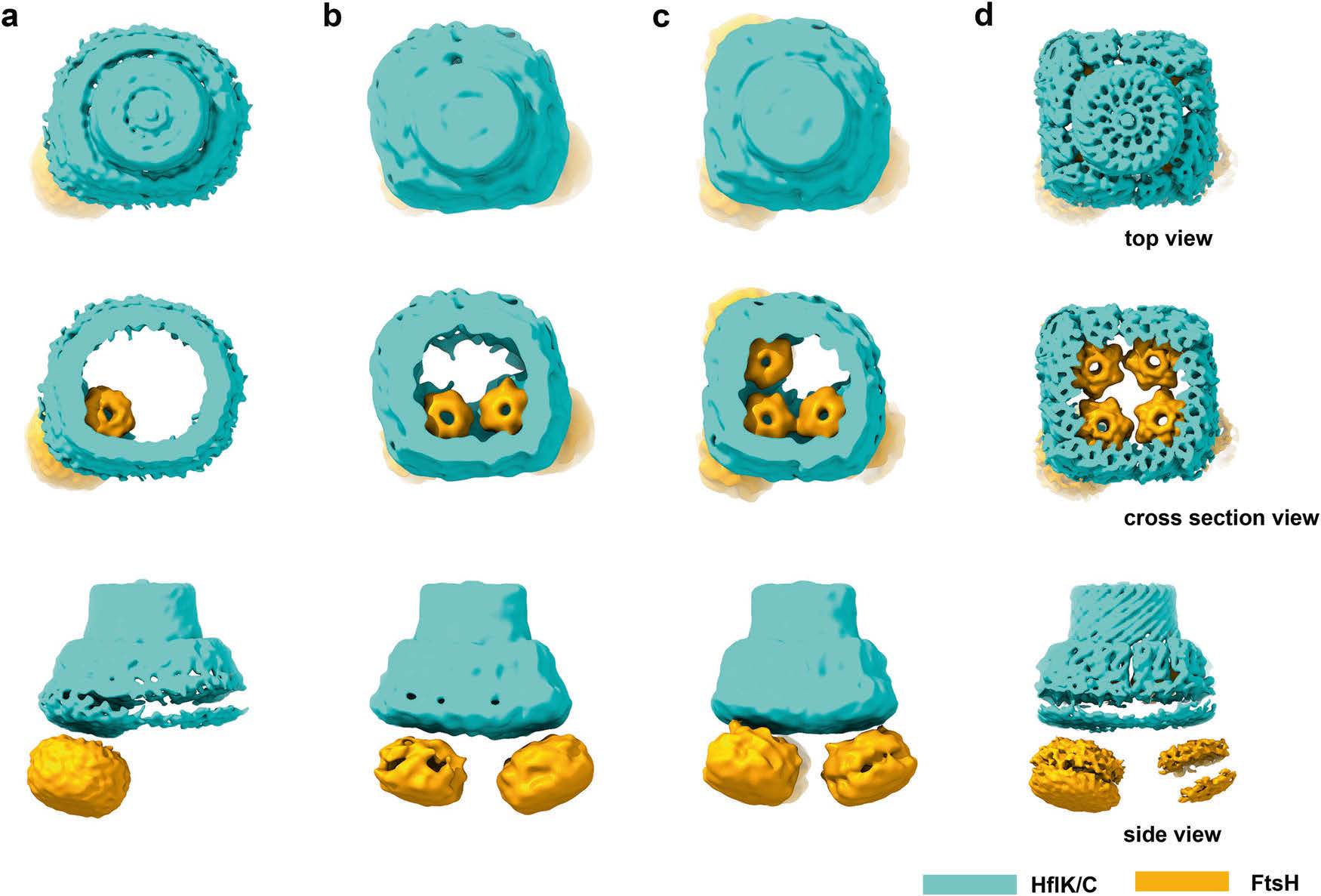
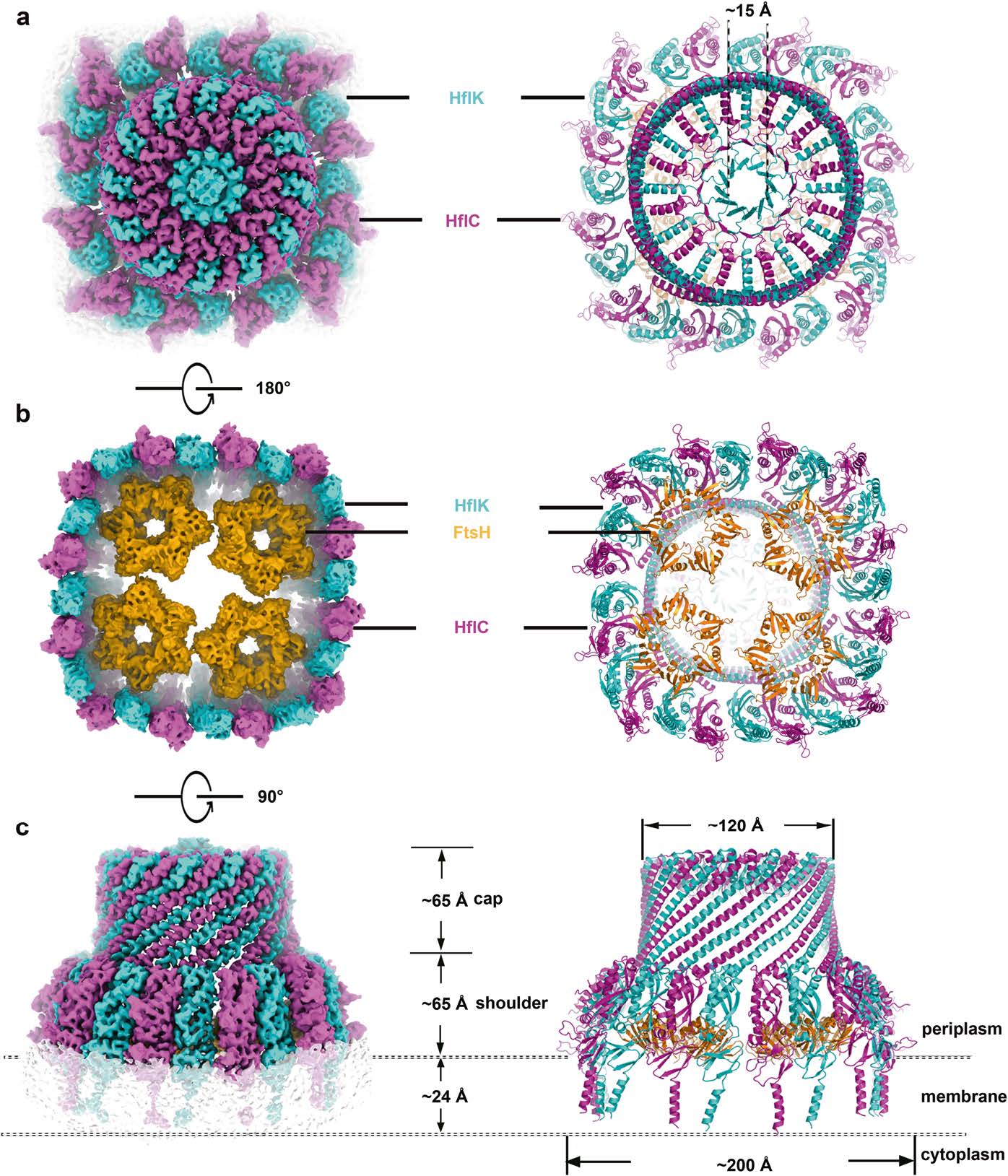
First of all, the Cryo-EM data analysis showed that HflK/C took heterodimer as the basic unit, and 12 HflK molecules and 12 HflC molecules were arranged at intervals on the intima to form a cage-like structure of transmembrane hetero-24 polymer. The transmembrane region (N-terminal region) of HflK/C formed and isolated a membrane microdomain (20 nm in diameter); most of the amino acid residues of HflK/C were located in the periplasmic space and assembled into a completely sealed cage structure. More interestingly, FtsH hexamers can be suspended in the micro membrane domain formed by HflK/C, and a cage structure can contain up to 4 FtsH hexamers. The periplasmic domain (PD) of FtsH hexamer was completely wrapped in the cage structure formed by HflK/C in the periplasmic space and directly interacted with the SPFH domain of HflK molecules in the complex.
Fig. 1 Purified KCF complexes are heterogenous in conformation and composition.
Secondly, based on the analysis of the high-resolution structure (3.3 Å) of the KCF complex containing four FtsH hexamers, some previously unknown common structure properties of the SPFH family were found. The SPFH domain peculiar to the SPFH family can be accurately divided into two domains (SPFH1 and SPFH2), in which the SPFH1 domain is actually a membrane insertion domain, and about half of the structure is inserted into the outer leaflet of the cell membrane; the adjacent SPFH1 domains interacted closely with each other, directly dividing a 20 nm diameter microdomain on the outer leaflet that was completely separated from the surrounding lipids. The structure clearly shows the complete oligomeric form of HflK and HflC interacting side by side from the N- terminal to the C- terminal: in addition to the interacting SPFH1 and SPFH2 domains, the domains (single α helix, CC1, and CC2) also interacted closely with each other, forming a cage-like wall through the right-handed helix. The domain closest to the C-terminal contains a characteristic β-strand (β 9) and all the HflK and HflC subunits in the complex contribute a β-strand, thus forming a stable β-barrel.
Fig. 2 The overall structure of the intact KCF complex.
The amino acid sequences involved in the recognition and binding of FtsH on the HflK subunit were further identified. Based on these structural information and biochemical experimental data, the molecular mechanism of HflK and HflC regulating bacterial membrane protein homeostasis through FtsH was proposed. Under normal physiological conditions, the cage structure of HflK/C can isolate FtsH proteases in space, thus preventing FtsH from degrading functional membrane proteins on the cell membrane, which is very important for maintaining the stability of membrane proteins on the overcrowded cell membrane. For abnormally assembled membrane proteins or damaged membrane protein complexes, the disordered sequence at the free end of the membrane protein complex can still be recognized by FtsH and selectively degraded.
Importantly, the structure of typical SPFH family proteins was analyzed based on sequence, and it was found that the domain organization of all SPFH family proteins was highly similar to that of HflK/C. These analyses suggest that all members of the SPFH family are likely to form cage-like structures on different subcellular membrane systems in a similar way, thus forming membrane microdomain of different sizes. In particular, it is worth pointing out that SPFH1, as a membrane insertion domain, is directly involved in the formation of leaflet-specific membrane microdomains. In view of the fact that different SPFH proteins have different membrane topological properties, SPFH has the ability to regulate the lipid fluidity of different leaflets. At the same time, the form of HflK/C cage structure recruiting and isolating FtsH hexamers also provides a novel model for understanding the functionalization of lipid microdomains: different SPFH proteins interact with membrane proteins with different functions (AAA+ proteases, ion channels, membrane receptors, etc.) anchored on the membrane to laterally isolate these functional molecules, thus giving functional specificity to the miniature membrane microdomains.
In summary, a very novel form of membrane protein complex was clarified by studying the structure and function of HflK and HflC of bacterial SPFH family, which is very important to understand the molecular mechanism of its mitochondrial homologous protein prohibitins in human-related diseases. Importantly, this work provides a “concrete” physical basis for understanding the “vague” concept of cell biology such as FMMs or lipid rafts. The cage structure of SPFH proteins on the membrane can perfectly explain some important scientific disputes in the existing lipid raft models, which will greatly promote the conceptual evolution in this field and provide a new starting point for the study on the whole SPFH protein family.