Tumor-associated inflammation (TAI) is an important hallmark of all cancers and involves all cells in the tumor microenvironment (TME), which describes the entire spectrum of cancer cells, immune cells, endothelial cells, and fibroblasts within a tumor. Despite its ubiquity in cancer, the consequences of TAI are highly context-dependent and double-edged. For example, prolonged inflammation in the liver or colon increases the risk of developing hepatocellular or colorectal cancer, respectively, and in established cancers, TAI promotes tumor formation.
On the other hand, TAI is required for tumor T-cell infiltration and is positively correlated with prognosis in many tumor entities. In addition, T cells are cellular mediators of immune checkpoint blockade (ICB), which means that ICB requires a certain level of TAI to function.
Recently, researchers from the Institute for Tumor Biology and Experimental Therapy in Frankfurt, Germany, published a review article entitled “Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity” in the journal Clinical Cancer Research, which reviews the role of tumor-associated fibroblasts in inflammation and antitumor immunity.
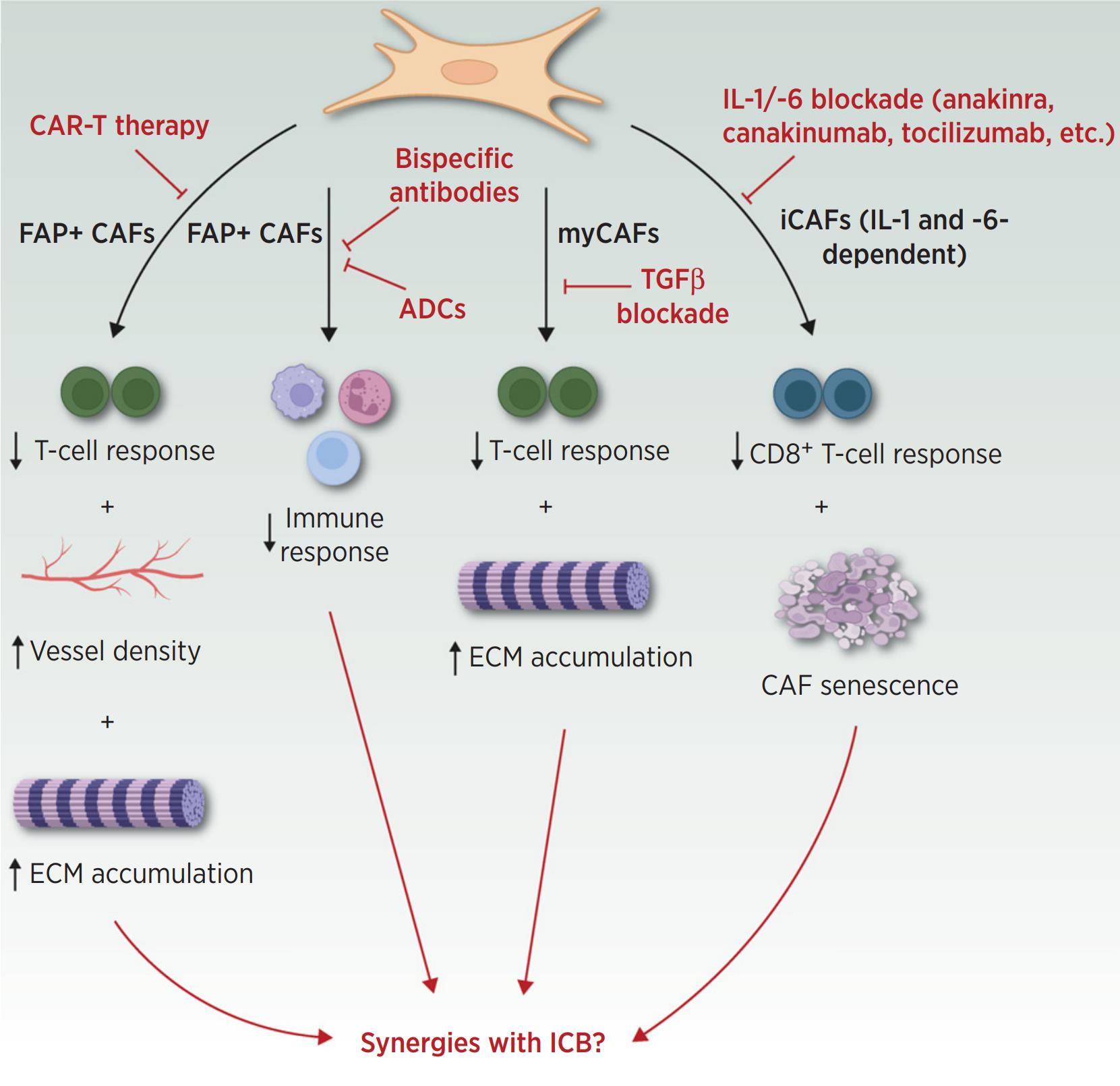
TAI is a feature of almost all cancers and has both tumor-promoting and tumor-suppressing functions. Tumor-associated fibroblasts (CAF) are a heterogeneous cellular component of the tumor microenvironment with a high degree of plasticity. Recent single-cell sequencing analyses have revealed distinct CAF isoforms in different human cancers and helped to identify key CAF isoforms such as myofibroblastic CAF, inflammatory CAF and antigen-presenting CAF, with the first two CAFs being present in almost all tumors.
Importantly, these three CAF populations are involved in and modulate the positive and negative consequences of TAI. The remarkable plasticity of CAFs allows them to change phenotypically and functionally in response to environmental changes. In this review, the investigators describe how CAFs promote tumor inflammation and suppress adaptive immune responses. The investigators also summarize recent evidence that has emerged regarding the suppression of tumor function by CAF in the context of TAI. Finally, the investigators summarize therapeutic concepts aimed at modulating CAF function or depleting immunosuppressive CAF to synergize with immunotherapy.
Over the past years, it has become evident that CAF cannot be considered as a pure tumor promoter in the context of TAI. In fact, CAFs represent a highly diverse and highly plastic cell population within the TME. Their multifunctional functions can be divided into “physical” and “chemical” aspects: CAFs “physically” block or promote immune cell infiltration by modulating the ECM, and “chemically” by secreting cytokines and chemokines to interact with immune cells.
Importantly, these soluble molecules are likely to be systemically active and therefore may affect metastatic spread. Hence these putative pleiotropic CAF effects, as well as other factors such as tumor entity, stage, TME composition, and prior therapeutic interventions, need to be carefully considered when developing therapeutic regimens aimed at modulating CAF function to enhance antitumor immune responses. However, their versatility and plasticity provide an excellent opportunity to modulate their respective functions for therapeutic purposes.
Reference
1. Kennel, Kilian B., et al. “Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity.” Clinical Cancer Research 29.6 (2023): 1009-1016.
