The field of drug discovery is witnessing a revolutionary shift with the advent of Proteolysis Targeting Chimeras (PROTACs). This innovative therapeutic strategy is redefining how we approach the degradation of proteins, especially protein kinases, which play a pivotal role in cellular signaling and cancer progression. The recent study by Sobierajski, Małolepsza, Pichlak, Gendaszewska-Darmach, and Błażewska published in Drug Discovery Today delves deep into the nuances of PROTAC design, specifically the critical role of E3 ligase selection in the degradation efficacy of protein kinases.
Understanding PROTACs
PROTACs are bifunctional molecules composed of two active domains: a warhead that binds the protein of interest (POI) and an E3 ligase ligand, connected by a linker. The successful formation of the ternary complex—comprising the POI, the PROTAC, and the E3 ligase—is crucial for the ubiquitination and subsequent proteasomal degradation of the target protein. The design intricacies of PROTACs, particularly the choice of E3 ligase, significantly influence their therapeutic efficacy and specificity.
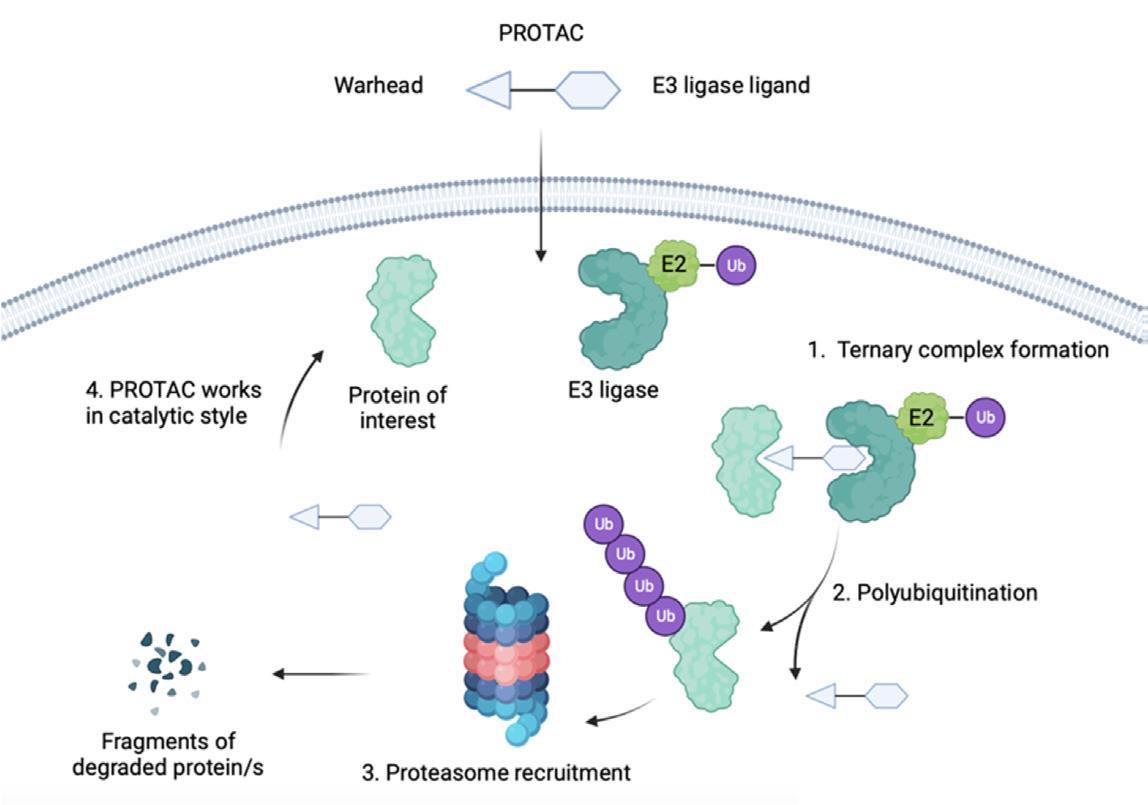
Fig.1 Mechanism of action of PROTA.1
Importance of E3 Ligase Selection
E3 ligases are enzymes that tag proteins with ubiquitin, marking them for degradation by the proteasome. The human genome encodes over 600 E3 ligases, but only a handful have been extensively used in PROTAC design, namely Von-Hippel-Lindau (VHL), cereblon (CRBN), mouse double-minute 2 homolog (MDM2), and inhibitor of apoptosis proteins (IAP). Each E3 ligase brings unique properties to the PROTAC mechanism:
Von-Hippel-Lindau (VHL): VHL is widely expressed and has been a popular choice due to its robust and predictable ubiquitination efficiency. VHL-based PROTACs have shown success in degrading kinases such as BRD4 and BTK.
Cereblon (CRBN): CRBN is known for its ability to induce the degradation of neosubstrates like IKZF1 and IKZF3, which is beneficial in anticancer strategies. However, CRBN ligands can also lead to off-target effects, necessitating careful design to minimize unwanted interactions.
MDM2: MDM2 targets the tumor suppressor p53, and its ligands can provide a dual therapeutic approach by both degrading the POI and activating p53 pathways.
IAP: IAP ligases have shown potential but are less commonly used due to challenges in identifying suitable ligands.
The study emphasizes that the degradation efficiency and specificity are not solely dependent on the E3 ligase but also on the linker’s properties, such as its length, rigidity, and polarity. The formation and stability of the ternary complex are influenced by the compatibility between the POI and the chosen E3 ligase, which can vary based on the cellular context and the specific target protein.
Recent Developments and Findings
CRBN-Based PROTACs: These have been effective in degrading a variety of kinases, but they also show a propensity for degrading off-target proteins, which can be therapeutically advantageous or detrimental depending on the context. For example, CRBN-based PROTACs derived from AKT inhibitors have shown efficacy in targeting PI3K/PTEN mutant cells but not KRAS/BRAF mutant cells.
VHL-Based PROTACs: These have demonstrated significant promise in degrading kinases such as ERK5 and CDK6. The review notes that VHL ligands tend to form more stable ternary complexes, leading to more efficient degradation of the POI.
Emerging E3 Ligases: The landscape of E3 ligase ligands is expanding, with new ligases like KEAP1 and DCAF15 being explored for their potential to target previously undruggable proteins.
Future Perspectives
The choice of E3 ligase is a critical determinant in the design and success of PROTACs. Future research aims to expand the catalog of E3 ligase ligands and understand their unique properties and interactions. There is also a growing interest in developing PROTACs that can target multiple ligases, providing a more versatile and robust degradation mechanism.
In conclusion, the strategic selection of E3 ligases, combined with precise linker design, holds the key to unlocking the full therapeutic potential of PROTACs. As the field evolves, the ability to harness diverse E3 ligases for targeted protein degradation will undoubtedly lead to more effective and personalized treatments for a wide range of diseases, particularly cancer.
Reference
- Sobierajski, Tomasz, et al. “The impact of E3 ligase choice on PROTAC effectiveness in protein kinase degradation.” Drug Discovery Today(2024): 104032.
