Chen, Po-Han, et al. “Modulation of phosphoprotein activity by phosphorylation targeting chimeras (PhosTACs).” ACS chemical biology 16.12 (2021): 2808-2815.
In the rapidly evolving field of drug development, targeting protein post-translational modifications (PTMs) has emerged as a promising avenue for treating various diseases, particularly cancers. Phosphorylation, a key PTM, plays a critical role in regulating many cellular processes, from cell growth to apoptosis. Dysregulated phosphorylation often leads to diseases, notably cancer and neurodegenerative disorders. Traditionally, therapies have focused on inhibiting kinases to control phosphorylation. However, these kinase inhibitors have limitations, such as drug resistance and off-target effects. A new approach—Phosphorylation Targeting Chimeras (PhosTACs)—is providing a novel way to address these challenges by selectively promoting protein dephosphorylation, offering a powerful alternative to kinase inhibitors.
Understanding Protein Phosphorylation and Dephosphorylation
Phosphorylation is a reversible PTM that occurs when a phosphate group is added to proteins, lipids, sugars, or metabolites, influencing their biological function. Kinases are the enzymes responsible for phosphorylation, while phosphatases remove phosphate groups through dephosphorylation. Though there are over 500 protein kinases, only 137 protein phosphatases have been identified, leading to an imbalance in the modulation of phosphorylation across biological systems.
Hyperphosphorylation, where proteins are excessively phosphorylated, can lead to diseases like Alzheimer’s and cancer. Tau protein hyperphosphorylation in Alzheimer’s disease is linked to microtubule dysfunction, while the retinoblastoma (Rb) tumor suppressor protein becomes inactivated due to hyperphosphorylation in various cancers. Kinase inhibitors, which prevent hyperphosphorylation by blocking kinases, have become widely used therapeutic tools. However, due to the broad roles that kinases play in signaling pathways, inhibiting them often leads to adverse off-target effects and the development of drug resistance.
In light of these challenges, scientists have turned to phosphatases as a more focused method of regulating phosphorylation. However, global activation of phosphatases can result in unwanted dephosphorylation across numerous pathways, leading to unintended consequences. This has spurred the development of PhosTACs, which selectively recruit phosphatases to specific target proteins for dephosphorylation, providing a more precise approach to control phosphorylation.
What Are PhosTACs?
PhosTACs (Phosphorylation Targeting Chimeras) are small, bifunctional molecules designed to promote dephosphorylation by facilitating the proximity between a phosphatase and a target protein. The concept is analogous to Proteolysis targeting chimeras, which degrade target proteins by recruiting E3 ligases. Instead of degradation, PhosTACs trigger the removal of phosphate groups from proteins, thus modulating their activity.
The proof-of-concept for PhosTACs was first demonstrated using the serine/threonine phosphatase PP2A, which regulates a majority of cellular phosphorylation events. PP2A is a holoenzyme consisting of three subunits: a catalytic subunit (PP2A C), a regulatory subunit (PP2A B), and a scaffold subunit (PP2A A). PhosTACs are designed to recruit the PP2A A subunit to the desired protein, enabling dephosphorylation.
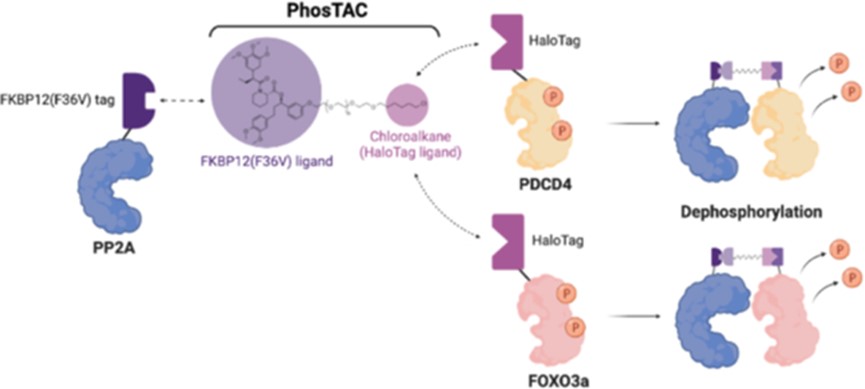
Fig.1 Schematic diagram of the mechanism of PhosTACs.1
PhosTACs in Action: Targeting PDCD4 and FOXO3a
The study detailed in the PDF explored the potential of PhosTACs using two tumor suppressor proteins: PDCD4 and FOXO3a. Both proteins are regulated by phosphorylation and play crucial roles in inhibiting oncogenic pathways.
- PDCD4 (Programmed Cell Death Protein 4): PDCD4 is phosphorylated by Akt and RSK, which leads to its inactivation and degradation. While the exact phosphatase responsible for dephosphorylating PDCD4 had not been previously identified, PhosTACs successfully recruited PP2A A, resulting in the dephosphorylation of PDCD4 at Serine 67 and Serine 457. This marked the first time targeted dephosphorylation of PDCD4 was achieved using a chemical biology approach.
- FOXO3a (Forkhead Box O3): FOXO3a is a transcription factor whose activity is suppressed by phosphorylation, particularly at Serine 318/321, which inhibits its ability to transcribe genes involved in apoptosis and cell cycle regulation. By recruiting PP2A A via PhosTACs, the study demonstrated that FOXO3a phosphorylation was significantly reduced, leading to the reactivation of its transcriptional activity. This shows the potential of PhosTACs to restore tumor suppressor function through targeted dephosphorylation.
Advantages of PhosTACs Over Traditional Kinase Inhibitors
One of the primary advantages of PhosTACs over kinase inhibitors lies in their mechanism of action. Kinase inhibitors typically work through an “occupancy-driven model,” where a small molecule binds to a kinase, preventing it from interacting with its substrates. However, this approach is stoichiometric—one inhibitor molecule can only affect one kinase molecule, and its effect is lost upon dissociation.
In contrast, PhosTACs function through an “event-driven model,” where a single PhosTAC molecule can induce the dephosphorylation of multiple target proteins by cycling through rounds of recruitment. This makes PhosTACs a potentially more effective tool, especially for conditions requiring prolonged modulation of protein phosphorylation.
Moreover, selectivity is another advantage of PhosTACs. Kinase inhibitors often have off-target effects due to the conserved ATP-binding sites across different kinases. PhosTACs, by focusing on recruiting phosphatases to specific substrates, reduce the risk of such off-target interactions.
Future Directions
While PhosTACs represent a promising new tool in the fight against diseases like cancer and neurodegenerative disorders, many questions remain. For instance, determining the most suitable phosphatases for different target proteins is a key challenge. Additionally, further research is needed to explore how PhosTACs can be used in vivo and whether they can overcome resistance mechanisms seen with kinase inhibitors.
The development of PhosTACs marks an exciting new frontier in precision medicine, offering the potential for targeted therapies that modulate protein function more selectively and efficiently than ever before. As the field advances, PhosTACs may become a staple in the arsenal of molecular tools used to combat a wide array of diseases.
Creative Biolabs is known for providing comprehensive services in proteolysis targeting chimeras molecule discovery. We assist with the design, synthesis, and screening of proteolysis targeting chimeras molecules, which are used for targeted protein degradation.
Browse our services:
Ligand Design for Target Protein
Ligand Screening for E3 Ligase
Linker Design and Optimization
Protein Degraders Structural Modification
Custom Peptide and Compound Synthesis
For more details regarding Creative Biolab’s Protein Degraders service, please feel free to contact us to assist you.
Reference
- Chen, Po-Han, et al. “Modulation of phosphoprotein activity by phosphorylation targeting chimeras (PhosTACs).” ACS chemical biology12 (2021): 2808-2815.
